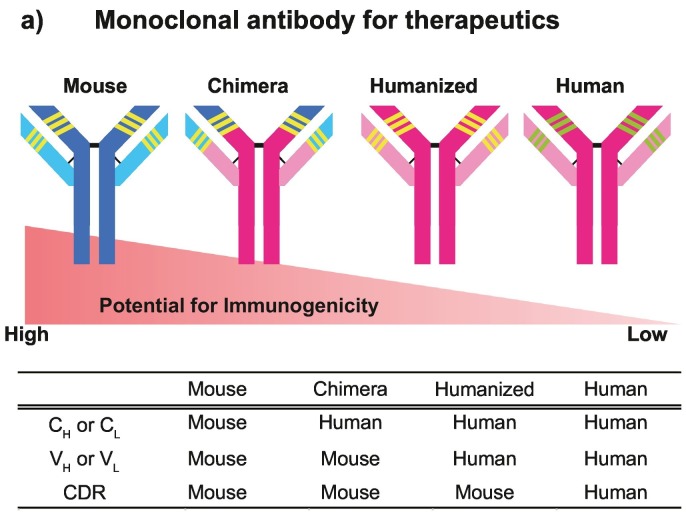
Fig. 1.
Potential for immunogenicity in therapeutic mAbs.
In a chimeric antibody, VH and VL (which contains the CDR) are the sequences derived from a mouse antibody; approximately 65% of the sequence is from a human antibody. On the other hand, a humanized antibody includes only the CDR of a mouse antibody. Over 90% of the antibody sequence is from human. CDR, complementarity determining region; CH and CL, constant regions of the heavy and light chains; VH and VL, variable regions of the heavy and light chains.