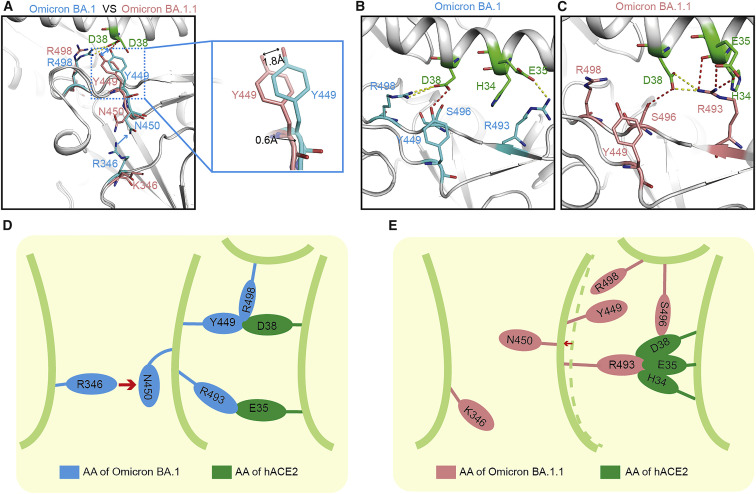
Figure 4.
A single substitution far away from the binding motif of BA.1.1 RBD changes the interaction network with hACE2
(A–C) Structural comparison between BA.1 RBD/hACE2 complex and BA.1.1 RBD/hACE2 complex. The complex structures are shown as a cartoon and the key amino acids are shown as sticks. The key amino acids in BA.1 RBD and BA.1.1 RBD are colored by cyan and salmon, correspondingly. The key residues in the hACE2 are colored green.
(D and E) Pattern diagram of R346K substitution in BA.1.1 RBD changing the interaction network. (D) Schematic of the conformation of the key amino acids in BA.1 RBD.
(E) Schematic of the conformation change of the key amino acids after R346K substitution in BA.1.1 RBD.