Abstract
The purpose of this study is to determine whether the levels of serum Sirt3 correlate with disease severity and perfusion indicators in septic patients, as well as to assess the clinical value of Sirt3 as a potential novel marker for sepsis diagnosis and mortality prediction. A total of 79 patients in the ICU were included in the study, of which 28 were postoperatively noninfectious and the remaining 51 patients were all diagnosed with sepsis during the study period. The levels of Sirt3 were detected and dynamically monitored by enzyme-linked adsorption method, Pearson or Spearman coefficient for correlation analysis between Sirt3 and clinical indicators, ROC curve for evaluation of diagnosis and mortality prediction, Kaplan–Meier method for the significance of Sirt3 in 28-day survival. The serum levels of Sirt3 were lower in the sepsis patients on day 1 (P < 0.0001), and the septic shock group had lower Sirt3 levels than the sepsis group (P = 0.013). Sirt3 had good negative correlations with SOFA scores both in sepsis and septic shock groups (Pearson: r2 = − 0.424, − 0.518; P = 0.011, 0.040), and Sirt3 correlated strongly with ScvO2 in the septic shock group (Pearson: r2 = − 0.679, P = 0.004) and with PCT in the sepsis group (Pearson: r2 = − 0.409, P = 0.015). Sirt3 not only performed well in identifying sepsis (AUC = 0.995, 95% CI 0.987–1, P < 0.0001) but also greatly enhanced lactate's specificity in detecting septic shock (from 91.43 to 94.29%). Patients in the low Sirt3 group had higher ScvO2, lactate, APACHE II score, SOFA score, longer ICU stays, and worse indicators of inflammation (TNF-α, IL-6) and infection (PCT) than those in the high Sirt3 group (P < 0.05). Additionally, Sirt3 can predict mortality of sepsis (AUC = 0.746, 95% CI 0.571–0.921, P = 0.022), patients with serum Sirt3 < 10.07 pg/ml have a lower 28-day survival (log-rank P = 0.008). Low serum levels of Sirt3 are significantly correlated with the disease severity. At the same time, Sirt3 increases the sensitivity of lactate to detect “cellular hypoxia” in septic shock. Sirt3 is a promising biomarker for the diagnosis of sepsis and predicting mortality risk in septic patients.
Subject terms: Diagnostic markers, Predictive markers, Prognostic markers
Introduction
Sepsis is a multi-organ failure that is caused by the body's dysregulated response to infection, and elevated serum lactate levels and circulatory failure constitute the core of septic shock1. Lactate levels, as well as their trends over time, are reliable indicators of the severity of sickness and mortality2,3. We now recognize that cytokine signaling4, mitochondria damage5, catecholamine stimulation6,7, and other factors8 can cause the rising of lactate in septic shock, and tissue hypoxia is no longer the main explanation of elevated lactate. Researchers have demonstrated that oxygen delivery to tissue is sufficient during sepsis, but increases in circulating lactate are still common in septic patients due to the limited ability of tissues to utilize oxygen. This hypoxia phenomenon caused by the inability of cells to utilize oxygen is called “Cytopathic hypoxia”9,10, which highlights the critical role of mitochondrial function in raising lactate levels during sepsis11.
Several pathways of mitochondria damage can lead to “Cytopathic hypoxia”. These involve damaging mitochondria by ROS from mitochondria themselves12, changing mitochondrial protein expression13,14, suppression of key enzymes in the tricarboxylic acid cycle (TCA)15, inhibition of mitochondrial enzyme complexes16–18, and exhaustion of the pyruvate dehydrogenase complex (PDC) activity19,20. In sepsis, mitochondrial dysfunction contributes to adverse outcomes, such as multiple organ dysfunction syndromes (MODS)21 and even death22.
Sirt3 is a NAD-dependent deacetylase found in mitochondrial-rich organs such as the kidney, heart, and brain23. Sirt3 can control the activity of metabolic enzymes such as succinate dehydrogenase (SDH)24, long-chain acyl-coenzyme A dehydrogenase (LCAD)25 to regulate the TCA26, oxidative phosphorylation27, and fatty acid metabolism pathways28 in mitochondria28. Sirt3 also plays a vital role in controlling the pattern of mitochondria function within cells, as well as the cell's adaptive response to metabolic stress29. The dysregulation of Sirt3 is also implicated in diseases involving mitochondria, such as cancer30, cardiac disease29,31, various metabolic disorders31, and aging32,33. Some studies have reported the clinical value of SIRT3 expression in various types of cancer34,35.
Similarly, Sirt3 also has been found in sepsis to protect various organs through its regulation of mitochondrial function, including inhibiting vascular inflammation36, protecting the pulmonary endothelial barrier function37, preventing cardiac insufficiency38,39, and also reducing renal40 and intestinal injury41. Sirt3 also contributes to regulating the immune metabolic switch between hyper-inflammatory and hypo-inflammatory during sepsis42. However, Sirt3 has been rarely studied in patients with sepsis. Based on the above background, we speculated that Sirt3 may be a useful marker for the recognition of “Cytopathic hypoxia” during sepsis. Therefore, in this prospective, non-interventional cohort study, we first described the levels of Sirt3 in the serum of patients with sepsis or septic shock, explored the correlation between serum Sirt3 levels and severity of illness, clinical perfusion indicators, and infection indicators, as well as the importance of Sirt3 as an indicator of diagnosing and predicting in septic patients.
Method
Design
The prospective observational clinical study was conducted at Peking Union Medical College Hospital from May 2021 to December 2021 which was approved by the PUMCH institutional review board (approval number JS-3302). We obtained informed consent from all enrolled patients through the next of kin of each patient. Our study was performed in accordance with the Declaration of Helsinki and was guided by the sepsis 3.0 diagnostic criteria and Clinical Nursing Practice Guidelines (2018) to ensure the safety of human subjects and sample compliance. We screened and categorized 79 patients within 24 h after their admission to the intensive care unit (ICU) based on strict inclusion and exclusion criteria, including 35 patients with sepsis, 16 patients with septic shock, and 28 patients without infection.
Patients
All patients in the study were older than 18 years. Patients with sepsis must meet the latest diagnostic criteria (Sepsis-3 defines sepsis as “life-threatening organ dysfunction caused by a dysregulated host response to infection; Sequential [Sepsis-related] Organ Failure Assessment Score (SOFA) is used to define organ dysfunction as an increase in the total SOFA score of 2 points or more)10. Patients with sepsis will be classified into the septic shock group when they meet the following criteria: persisting hypotension requiring vasopressors to maintain mean arterial pressure [MAP] > 65 mmHg and having serum lactate level > 2 mmol/L despite adequate volume resuscitation. Patients in the ICU control group need to have normal infection indicators.
The enrolled septic patients were assessed on each study day to determine if they remained in sepsis or septic shock. During the study, patients who were transferred from ICU due to abandonment of treatment or improvement of their condition, as well as those who were no longer diagnosed with sepsis in the ICU, were not studied on subsequent study days (D3, D5, or D7).
Patients who had been or were currently diagnosed with cancer, including all types of tumors, were excluded from the study. Patients who are pregnant were also excluded.
Serum collection
We collected arterial blood through an arterial indwelling catheter. Septic patients were collected on days 1, 3, 5, and 7 after being admitted to the ICU, while patients in the control group were only collected on day 1. Blood collected in the anticoagulant tube was centrifuged at 3000 r/min for 10 min after standing for 10 min, then transferred to an EP tube and stored at − 80 °C.
Clinical data collection
The patient's admission information (age, gender, primary disease, history, infection site, Length of hospital stay, Length of ICU stay), basic vital signs (blood pressure, heart rate, respiratory rate, body temperature), biochemical examination (white blood cells, hemoglobin, PCT, IL-6, IL-10, hs-CRP), SOFA, APACHE II, ventilator parameters, antibiotics, vasoactive drugs, lactate, central venous oxygen saturation (ScvO2), and other information were also collected.
Elisa for serum Sirt3 level
After defrosting the frozen serum, we used the human Sirt3 ELISA kit (Cusabio Biotech Co, Ltd, Wuhan, China, Catalog # CSB-EL021341HU) to detect the concentration of Sirt3 in the serum.
Statistical analysis
We used SPSS 26.0 and GraphPad PRISM 8.0 for statistical analysis and plotting. The Shapiro–Wilk test tested whether the data were normally distributed. The normally distributed data were expressed as mean ± standard, and compared by the One-way Anova or T-test. Non-normal distribution data were expressed as M (P25, P75), and compared by the Kruskal–Wallis test or Mann–Whitney U test. And a variance homogeneity test was also performed, and non-parametric tests were used when variances are uneven. The counting data were expressed as rates (%), and the comparison between groups was tested by χ2. A Pearson correlation coefficient analysis was for normally distributed data, a Spearman rank correlation coefficient analysis was for non-normally distributed data. ROC curves were used to analyze the diagnostic efficacy of various indicators. 28-day survival was estimated by the Kaplan–Meier method, and differences in survival were evaluated with the log-rank test. P < 0.05 means that the difference between the two groups is statistically significant.
Results
Baseline characteristics
A total of 79 patients were included in this study, 51 of whom were diagnosed with sepsis during the study period, and 28 were non-infected patients postoperatively. We obtained baseline data on three groups of patients (Table 1) after grouping by diagnosis on day 1. There were 35 patients in the sepsis group (57.88 ± 20.78 years, 60% male), 16 in the septic shock group (64.06 ± 17.02, 75% male), and 28 in the ICU control group (58.25 ± 20.70, 60.7% male). The past medical history was counted in all groups (sepsis VS septic shock VS ICU control), including hypertension (31.4% vs. 56.25% vs. 46.43%), diabetes mellitus (22.9% vs. 18.75% vs. 25%), hyperlipidemia (11.4% vs. 6.25% vs. 17.86%), heart attack (11.4% vs. 18.75% vs. 39.29%), cerebral hemorrhage (5.7% vs. 6.25% vs. 10.71%). The sites of infection of patients in the sepsis group and septic shock group were also counted: lung (20% vs. 6.25%), abdomen (34.29% vs. 50%), central nervous system (11.42% vs. 12.5%) bloodstream infection (14.29% vs. 12.5%), skin (5.7% vs 6.25%) or others (14.29% vs 12.5%). According to the follow-up of 28-day mortality, 9 septic patients died with a mortality rate of 17.65%, including 4 in the sepsis group (11.42%) and 5 in the septic shock group (37.5%).
Table 1.
Baseline characteristics of all patients on day 1.
| Sepsis (n = 35) | Septic shock (n = 16) | ICU control (n = 28) | P value | |
|---|---|---|---|---|
| Sex [male] (%) | 21 (60%) | 12 (75%) | 17 (60.7%) | |
| Age (years) | 57.88 ± 20.78 | 64.06 ± 17.02 | 58.25 ± 20.70 | 0.587 |
| MAP (mmHg) | 81.58 ± 10.94 | 84.38 ± 6.84 | 86.36 ± 10.36 | 0.173 |
| HR (bpm) | 89.09 ± 17.836 | 100.44 ± 14.02 | 78.18 ± 15.38 | < 0.05 |
| CVP (cmH2O) | 6.91 ± 2.17 | 9.12 ± 2.19 | – | < 0.05 |
| past medical history (n, %) | ||||
| Hypertension | 11 (31.4%) | 9 (56.25%) | 13 (46.23%) | |
| Diabetes | 8 (22.9%) | 3 (18.75%) | 7 (25%) | |
| Hyperlipidemia | 4 (11.4%) | 1 (6.25%) | 5 (17.86%) | |
| Cardiovascular disease | 4 (11.4%) | 3 (18.75%) | 11 (39.29%) | |
| Cerebral infarction | 2 (5.7%) | 1 (6.25%) | 3 (10.71%) | |
| Site of infection (n, %) | ||||
| Pneumonia | 7 (20%) | 1 (6.25%) | – | |
| Peritonitis | 12 (34.29%) | 8 (50%) | – | |
| Central system infection | 4 (11.42%) | 2 (12.5%) | – | |
| Bloodstream infection | 5 (14.29%) | 2 (12.25%) | – | |
| Skin infections | 2 (5.7%) | 1 (6.25%) | – | |
| Others | 5 (14.29%) | 2 (12.25%) | – | |
| SOFA score | 9.37 ± 3.34 | 13.35 ± 2.828 | 1.57 ± 2.659 | < 0.05 |
| APACHE II score | 18 ± 7.68 | 21.5 ± 7.95 | 13.5 ± 6.46 | < 0.05 |
| NE (ug/kg/min) | 0.049 (0,0.568) | 0.798 (0.071,1.15) | – | < 0.05 |
| Lactate (mmol/L) | 1.2 (0.6,2.6) | 3.5 (2.1,9.7) | 0.6 (0.8,3) | < 0.05 |
| P(V-A)CO2 (mmHg) | 2.9 (1.65,4.6) | 3.2 (1.25,4.88) | 0.828 | |
| ScvO2 (%) | 74.17 ± 8.14 | 75.87 ± 10.03 | – | 0.54 |
| OI | 314.58 ± 125.46 | 268.41 ± 118.83 | 374.70 ± 132.47 | < 0.05 |
| PCT (ng/ml) | 15.34 ± 23.45 | 16.97 ± 23.65 | 0.67 ± 1.29 | < 0.05 |
| WBC (109/L) | 14.24 ± 7.68 | 10.68 ± 9.07 | 11.78 ± 2.55 | 0.155 |
| TNF-α (mg/L) | 17.49 ± 12.84 | 30.80 ± 15.71 | – | < 0.05 |
| IL-10 (pg/ml) | 7.35 (5.175,109.5) | 15.6 (7.24,52.3) | – | 0.108 |
| IL-6 (pg/ml) | 136.49 ± 267.47 | 446.41 ± 87.45 | – | < 0.05 |
| CTNI (ng/ml) | 5.32 ± 21.38 | 40.64 ± 151.21 | 0.18 ± 0.02 | 0.402 |
| NT-proBNP (pg/ml) | 6075.47 ± 9225.54 | 7972.45 ± 10,137.53 | 893.85 ± 700.20 | 0.244 |
| Length of ICU stay (days) | 8 (3.5,8.75) | 12 (8,43) | 1 (1,17) | < 0.05 |
| Length of hospital stay (days) | 27 (5,121) | 34.5 (3,88) | 9 (3,38) | < 0.05 |
| 28-day mortality rate (n,%) | 4 (11.42%) | 5 (37.5%) | 0 | < 0.05 |
Values are expressed as mean ± standard deviation, or median (interquartile range), or number (percentage). P < 0.05 were considered statistically significant.
MAP mean arterial pressure, HR heart rate, CVP Central venous pressure, SOFA Score Sequential Organ Failure Assessment Score, APACHE II score Acute Physiology and Chronic Health Evaluation II score, NE Norepinephrine, P(V-A)CO2 central venous-to-arterial carbon dioxide difference, ScvO2 Central venous oxygen saturation, OI oxygenation index, PCT procalcitonin, WBC white blood cell, TNF-α Tumor Necrosis Factor α, IL-10 Interleukin-10, IL-6 Interleukin-6, CTNI cardiac troponin I, NT-proBNP N-terminal (NT)-prohormone Brain natriuretic peptide.
During the assessment of the patients on the first day, vital signs (heart rate, blood pressure), infection indicators (WBC, PCT), inflammatory indicators (TNF-α, IL-10, IL-6), CVP, Norepinephrine (NE), oxygenation index (OI), CTNI, and NT-pro BNP were collected. Except for MAP, P(V-A)CO2, ScvO2, WBC, IL-10, CTNI, and NT-pro BNP, the rest of the indicators were statistically significant (Table 1).
Characteristics of changes in serum levels of Sirt3 in patients with ICU
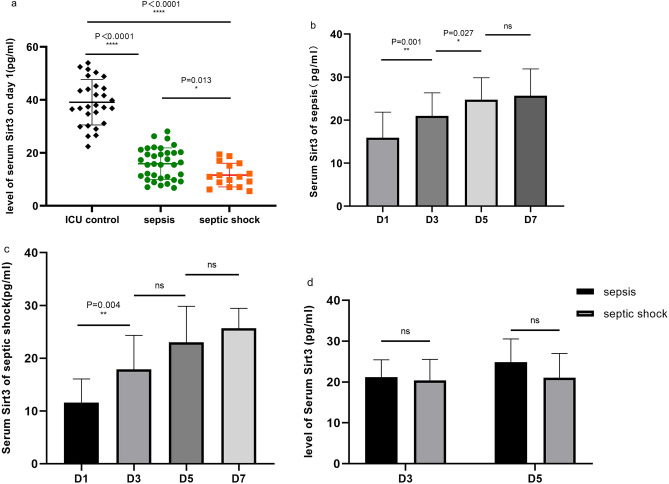
The serum level of Sirt3 was lower in septic patients on day 1 (P < 0.0001), and the septic shock group had a lower level than the sepsis group (P = 0.013) (Fig. 1a).
Figure 1.
Characteristics of changes in serum levels of Sirt3 in patients with ICU. (a) Serum Sirt3 levels were measured in sepsis group, septic shock group, and ICU control group. (b) Serum Sirt3 levels on day1, day3, day5, and day7 in patients diagnosed with sepsis on day 1. (c) Serum Sirt3 levels on day1, day3, day5, and day7 in patients diagnosed with septic shock on day 1. (d) Serum Sirt3 levels were measured in sepsis group and septic shock group after re-diagnosed on D3 and D5. P < 0.05 were considered statistically significant. *denotes P < 0.05, **denotes P < 0.01, ****denotes P < 0.0001, “ns” means no significance.
We tracked changes in Sirt3 serum levels on days 3, 5, and 7. The Sirt3 levels in the sepsis group gradually increased over time, and there were statistical differences in growth between D3 and D1 (P = 0.001), D5 and D3 (P = 0.027), but there was no difference between D5 and D7 (P = 0.671) (Fig. 1b). A gradual increase in Sirt3 levels over time was also observed in the septic shock group, where there was a statistical difference between D3 and D1 (P = 0.004), but no statistical difference between D5 and D3, D7 and D5 (P = 0.052, 0.324) (Fig. 1c).
We re-diagnosed and re-grouped the patients on D3, D5, and D7 to confirm that the diagnosis was as accurate as feasible because the patients' conditions may interchange after therapy. On D3, 10 patients were transferred from the ICU, and 41 patients were studied, including 30 patients with sepsis (25 patients with sepsis and 5 patients with septic shock at D1), 11 patients with septic shock (2 patients with sepsis and 9 patients with septic shock at D1). On the fifth day, 11 patients left the ICU, and the remaining 30 patients included 23 patients with sepsis (18 patients with sepsis and 5 patients with septic shock at D3) and 7 patients with septic shock (2 patients with sepsis and 5 patients with septic shock at D3). On D7, 11 patients were moved from the ICU and 19 patients remained, including 17 patients with sepsis (14 with sepsis and 3 with septic shock from D5) and 2 with septic shock (from the septic shock group at D5). The difference in Sirt3 in the two groups was then compared again on days 3 and 5, but there was no statistical difference (Fig. 1d). Since there were only 2 patients in the septic shock group on D7 after being re-diagnosed, the difference between the two groups had a low confidence level and was therefore not shown.
Correlation between Sirt3 and clinical indicators in ICU patients
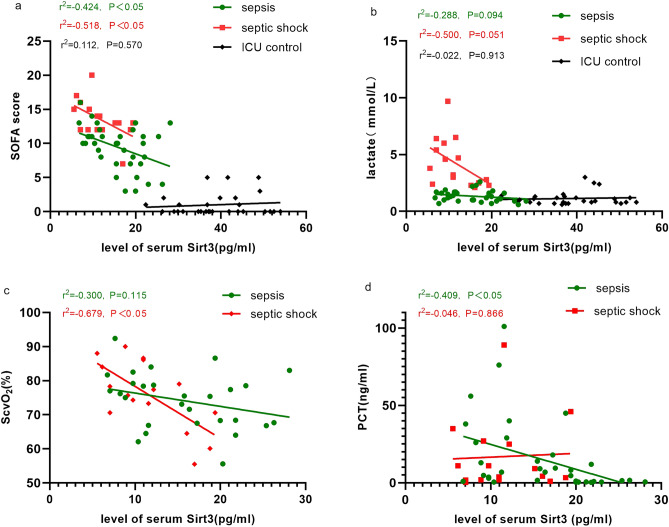
Our analysis found significant differences in Sirt3 levels among the three groups of patients on day 1, so we analyzed the correlation between Sirt3 levels and clinical indicators among the three groups on day 1.
Our first analysis was to determine if Sirt3 correlated with organ dysfunction, and we found that Sirt3 had a negative correlation with SOFA scores both in sepsis and septic shock groups (Pearson: r2 = − 0.424, − 0.518; P = 0.011, 0.040), but no significant correlation with the ICU controls (Pearson: r2 = 0.112, P = 0.570) (Fig. 2a). No correlation was found between Sirt3 and APACHE II scores in any of the three groups (Pearson: r2 = − 0.381, − 0.064, − 0.075 P = 0.143, 0.713, 0.705).
Figure 2.
Correlation between Sirt3 and clinical indicators in ICU patients. (a) Correlation between serum Sirt3 and SOFA score in sepsis group, septic shock group, and ICU control group. (b) Correlation between serum Sirt3 and lactate in sepsis group, septic shock group, and ICU control group. (c) Correlation between serum Sirt3 and ScvO2 in sepsis group and septic shock group. (d) Correlation between serum Sirt3 and PCT in sepsis group and septic shock group. P < 0.05 were considered statistically significant. SOFA Sequential Organ Failure Assessment Score, ScvO2 Central venous oxygen saturation, PCT procalcitonin.
The correlation analysis between Sirt3 and clinical indicators of perfusion revealed that Sirt3 did not correlate with Lactate in the three groups (Spearman: r2 = − 0.500, − 0.288, 0.022 P = 0.051, 0.094, 0.913) (Fig. 2b). However, Sirt3 correlated well with ScvO2 in the septic shock group of patients (Pearson: r2 = − 0.679, P = 0.004), but had no correlation with the sepsis group (Pearson: r2 = − 0.300, P = 0.115) (Fig. 2c). Similarly, Sirt3 did not correlate with P(V-A)CO2 in either group (Spearman: r2 = − 0.084, − 0.197; P = 0.664, 0.46). Data for ScvO2 and P(V-A)CO2 were not obtained because central venous access was not established in patients in the ICU control group.
The correlation between serum Sirt3 and PCT was examined in the groups with sepsis (Pearson: r2 = − 0.409, P = 0.015), but not in the groups with septic shock (r2 = 0.046, P = 0.866) (Fig. 2d).
The potential value of Sirt3 in the diagnosis of sepsis and septic shock
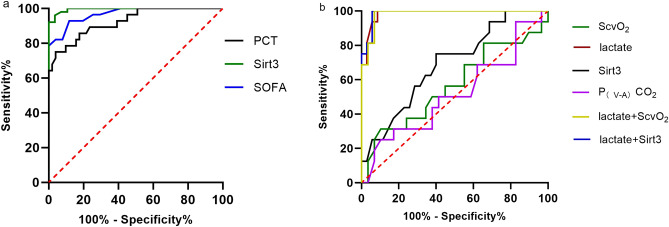
The ROC curves of Sirt3 for the diagnosis of sepsis and septic shock were also displayed. Sirt3 performed better in identifying sepsis (AUC = 0.995, 95% CI 0.987–1, P < 0.0001) than SOFA (AUC = 0.968, 95% CI 0.934–0.1, P < 0.0001), PCT (AUC = 0.925, 95% CI 0.865–0.985, P < 0.0001) (Fig. 3a). And based on the cutoff value of 25.85 pg/ml Sirt3 yielded sensitivity and specificity were 96.08% and 96.43%.
Figure 3.
The ROC curves of indicators for the diagnosis of sepsis and the early detection of sepsis from septic shock. (a) The ROC curves of indicators for the diagnosis of sepsis (PCT, Sirt3, SOFA). (b) The ROC curves of indicators for the early detection of septic shock (lactate, Scvo2, Sirt3, lactate + Sirt3, Lactate + ScvO2, P(V-A)CO2). Calculate the predicted values for lactate + Sirt3 and lactate + ScvO2 by binary logistic regression and then calculate the ROC curve. SOFA Sequential Organ Failure Assessment Score, ScvO2 Central venous oxygen saturation, PCT procalcitonin, P(V-A)CO2 central venous-to-arterial blood carbon dioxide partial pressure.
In screening septic shock patients from septic patients, we discovered that Sirt3 (AUC = 0.69, 95% CI 0.537 − 0.843, P = 0.031) has better performance than ScvO2 and P(V-A)CO2 (Fig. 3b, Table2), and lactate's specificity in detecting septic shock could be greatly enhanced (from 91.43% to 94.29%) when lactate was utilized in conjunction with Sirt3 (Fig. 3b, Table2).
Table 2.
The AUC and optimal study parameter cutoff points for different indicators of septic shock and their associated diagnostic and efficacy values.
| AUC ± SE | P-value | Cut-off value | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| Lactate | 0.986 ± 0.012 | < 0.001 | 2 (mmol/l) | 100% | 91.43% |
| ScvO2 | 0.567 ± 0.094 | 0.46 | 83.5 (%) | 31.25% | 89.66% |
| Sirt3 | 0.690 ± 0.078 | < 0.05 | 15.33 (pg/ml) | 75% | 60% |
| P(V-A)CO2 | 0.518 ± 0.094 | 0.84 | 1.25 (mmHg) | 25% | 89.66% |
| Lactate + Sirt3 | 0.988 ± 0.011 | < 0.001 | – | 100% | 94.29% |
| Lactate + ScvO2 | 0.982 ± 0.015 | < 0.001 | – | 100% | 93.1% |
ScvO2 Central venous oxygen saturation, P(V-A)CO2 central venous-to-arterial carbon dioxide difference. AUC the area under the ROC curve; Cut-off value the optimal cutoff points for the sample diagnosis of septic shock. P < 0.05 means statistically significant.
Difference of clinical indicators in different serum levels of Sirt3
We got a cutoff value (15.33 pg/ml) from the maximal Youden index of the ROC curve for screening septic shock. We divided the patients into low Sirt3 (< 15.33 pg/ml) and high Sirt3 (≥ 15.33 pg/ml) groups, with 25 patients in the low Sirt3 group (13 sepsis and 12 septic shock) and 26 patients in the high Sirt3 group (22 sepsis and 4 septic shock). After comparing and assessing the differences in clinical indicators between the two groups, we discovered that patients in the low Sirt3 group had higher APACHE II and SOFA scores, longer ICU stays, worse indicators of inflammation (TNF-α, IL-6) and infection (PCT) than those in the high Sirt3 group (Table3).
Table 3.
Comparative clinical data grouped by cutoff value (15.33 pg/ml) in patients with sepsis.
| Low Sirt3 (n = 25) | High Sirt3 (n = 26) | P value | |
|---|---|---|---|
| HR (bpm) | 94.8 ± 17.98 | 90.57 ± 16.97 | 0.392 |
| MAP (mmHg) | 84.04 ± 10.53 | 80.92 ± 9.11 | 0.263 |
| CVP (cmH2O) | 7.48 ± 2.16 | 7.84 ± 2.57 | 0.585 |
| OI | 305.18 ± 109.72 | 295.21 ± 138.59 | 0.778 |
| HB (g/l) | 99.68 ± 31.21 | 98.85 ± 22.83 | 0.913 |
| RASS | − 1.96 ± 1.43 | − 2.58 ± 1.55 | 0.372 |
| NE (ug/kg/min) | 0.23 (0.056,0.49) | 0.096 (0,0.231) | 0.203 |
| PEEP (ml/cmH2O) | 5 (5,6.75) | 5 (4.5,8) | 0.873 |
| Diagnosis (n,%) | |||
| Sepsis | 13 (52%) | 22 (84.6%) | |
| Septic shock | 12 (48%) | 4 (15.4%) | |
| Severity of illness | |||
| SOFA score | 12.84 ± 2.68 | 8.69 ± 3.27 | < 0.001*** |
| APACHE II score | 21.2 ± 7.97 | 16.8 ± 7.00 | 0.045* |
| Length of ICU stay (days) | 8 (5,10) | 14 (8,29) | 0.035* |
| Length of hospital stay (days) | 26 (14.5,40.75) | 27.5 (14,42.25) | 0.713 |
| Perfusion indicators | |||
| P(V-A)CO2 (mmHg) | 2.5 (1.625,4.775) | 3.5 (1.45,4.6) | |
| Lactate (mmol/l) | 1.9 (1.5,4.6) | 1.25 (1,2.025) | 0.003* |
| ScvO2 (%) | 79.16 ± 6.86 | 69.78 ± 8.14 | < 0.001*** |
| Laboratory parameters of infection | |||
| PCT ( (ng/ml)) | 68 (13,87) | 2.45 (0.675,9.4) | 0.019* |
| Hs-CRP (mg/l) | 190 (188.41,190) | 190 (190,190) | 0.865 |
| WBC (109) | 11.37 ± 7.52 | 13.62 (7.8,17.05) | 0.137 |
| Inflammatory cytokines | |||
| TNF-α (mg/l) | 24.56 (18.35,34.15) | 24.37 (12.87,24.55) | 0.033* |
| IL-10 (pg/ml) | 75.53 (15.6,86.763) | 35 (6.15,86.76) | 0.144 |
| IL-8 (pg/ml) | 278.5 (85.25,366) | 197 (74.75,366) | 0.315 |
| IL-6 (pg/ml) | 209.6 (108,244) | 137.5 (39.73,209.6) | 0.024* |
Values are expressed as mean ± standard deviation, median (interquartile range), or number (percentage).*denotes P < 0.05, ***denotes P < 0.001
MAP mean arterial pressure, HR heart rate, CVP Central venous pressure, OI oxygenation index, SOFA Score Sequential Organ Failure Assessment Score, APACHE II score Acute Physiology and Chronic Health Evaluation II score, P(V-A)CO2 central venous-to-arterial carbon dioxide difference, HB Hemoglobin, NE Norepinephrine, RASS Richmond Agitation-Sedation Scale, P(CV-A)CO2 central venous-to-arterial blood carbon dioxide partial pressure, ScvO2 Central venous oxygen saturation, OI oxygenation index, PCT procalcitonin, Hs-CRP high-sensitivity C-reactive protein, WBC white blood cell, TNF-α Tumor Necrosis Factor α, IL-10 Interleukin-10, IL-8 Interleukin-8, IL-6 Interleukin-6.
Mostly, It’s interesting that even though parameters impacting ScvO2 and lactate (RASS score, hemoglobin, OI, heart rate, P(V-A)CO2, blood pressure, CVP, NE) did not differ, ScvO2 and lactate showed significant difference between the two groups (p < 0.001) (Table3).
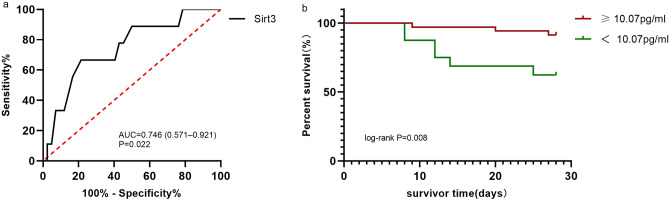
Predictive value of Sirt3 for 28-day survival
A total of 9 patients died during the 28-day follow-up period in our research, we plotted the ROC curve for Sirt3 predicted mortality. The area under the curve for Sirt3 was 0.746 (95% CI 0.571–0.921, P = 0.022, Fig. 4a). Based on the cutoff value of 10.07 pg/ml of Sirt3 yielded sensitivity and specificity were 66.67% and 78.57%, and all the patients were divided into two groups.
Figure 4.
The ROC curves of predicting for 28-day survival and Kaplan–Meier estimator analysis of Sirt3 for 28 day survival. (a) The ROC curves of Sirt3 for the predicting 28-day mortality in patients with sepsis. P < 0.05 were considered statistically significant (b) Kaplan–Meier estimator analysis of Sirt3 for 28-day survival. The cut off value of Sirt3 for the estimated 28-day mortality of patients with sepsis was 10.07 pg/ml.
Kaplan–Meier estimator analysis showed the 28-day survival rate of patients with low Sirt3 was markedly reduced than that of patients with high Sirt3 (log-rank P = 0.008, Fig. 4b).
Discussion
In our research, Sirt3 showed good clinical significance in sepsis. Sirt3 correlated with PCT in the sepsis group and was substantially linked with Scvo2 in the septic shock group, and its levels were negatively correlated with illness severity. Sirt3 also improved the specificity of lactate in the diagnosis of septic shock. It was an acceptable diagnostic marker for identifying sepsis and predicting mortality risk in septic patients. Furthermore, patients with low levels of Sirt3 had a worse prognosis and lower 28-day survival.
Sirt3 is a member of the Sirtuins family, which is composed of seven highly conserved NAD-dependent deacetylases that could regulate cell survival43, metabolism44, and longevity45. Our research showed a strong correlation between Sirt3 and SOFA, which is consistent with the results of most basic experiments on sepsis-induced MODS. In the study of septic cardiomyopathy, Sirt3 can activate AMPK-related mitochondrial biogenesis39 and acetylate important enzymes in the tricarboxylic acid cycle29 to reduce sepsis-induced myocardial injury in mice. Sirt3-AMPK signaling is also important for suppressing vascular inflammation and endothelial dysfunction during early sepsis36. Lack of Sirt3 increases CLP-induced renal insufficiency, tubular cell death and apoptosis, mitochondrial dysfunction, and ROS production in a knockout mouse model of AKI46. These studies focused on the cellular energy metabolism pathway have helped us understand Sirt3's role in protecting organ function at the cellular level, and our research demonstrated a close correlation between the serum level of Sirt3 and MODS in sepsis patients. MODS has been suggested to be caused by mitochondrial damage and energy metabolism disorders in cells during sepsis47, thus producing a phenomenon similar to “hibernation”48, which closely joins energy metabolism to MODS. Sirt3 contains the switches that control mitochondrial energy metabolism, it may be a potential strategy for future sepsis treatments that target mitochondria.
As one of the diagnostic criteria for septic shock, lactate is used as a perfusion indicator49 and a resuscitation therapy endpoint50 in clinical practice, although it can also signify numerous causes of non-perfusion. No correlation between Sirt3 and lactate was found in our study, and the reasons for this result may include small sample size and a complex mechanism of lactate elevation. Lactate is a ubiquitously produced and utilized metabolite that has a core place in nearly all energy-related pathways in humans. There is no consensus on the source, clearance, and metabolic functions of lactate in sepsis, and even we are not certain if high levels of lactate provide protection in sepsis. Understanding the pathophysiology and clinical significance of lactate is difficult. Hyperoxia is no longer a complete explanation for elevated lactate in sepsis, and mitochondrial dysfunction and decreased PDC activity have also been questioned. Research indicated51 that the early elevation in muscle lactate concentration during LPS infusion was not attributable to the mitochondrial dysfunction or PDC inhibition. Even if the mitochondrial function is normal, lactate levels appear to rise when pyruvate generation surpasses PDC to Acetyl Coenzyme A conversion. Elevated lactate is more likely to be a metabolic stress response under stress rather than just the result of cellular oxygenation problems in sepsis. Faced with the complexity of lactate, Sirt3, an indicator of regulating mitochondrial metabolism, is difficult to achieve desirable results in studies with small sample sizes. We still hope to have a large sample study in the future to investigate the correlation between Sirt3 and lactate.
Even though the cause of lactate production is not sufficiently clear, mitochondrial dysfunction remains a significant element in the formation of increased lactate during the septic shock phase. Lactate responds too slowly to be used to guide acute changes in sepsis52, Sirt3 may be an earlier indicator of the phenomenon of cellular hypoxia than lactate. So we tried to explore the potential of Sirt3 for identifying patients at risk of developing septic shock whose lactate cannot be distinguished from sepsis timely, but unfortunately, due to only 2 sepsis patients progressed to septic shock when we regrouped (on D3 and D5) during the study, we did not get such results. We may need to find a larger sample size to verify again. But we found that Sirt3 compensates for lactate deficiency in response to the phenomenon of "cytopathic hypoxia" in septic shock. Sirt3 allows high levels of circulating lactate as an indicator of impaired cellular oxygen metabolism.
ScvO2 is an indicator of the balance between oxygen supply and oxygen consumption, and it can be easily disturbed by several factors, such as mitochondria, hemoglobin, cardiac output, oxygen partial pressure, and patient agitation. But ScvO2 cannot provide a clear explanation for the imbalance of systemic oxygen supply and demand, which is the limitation of ScvO2, especially during septic shock. Current research suggests that impaired microcirculation and mitochondria function cause both an insufficient supply and ineffective utilization of oxygen, and these two factors affect tissue oxygen utilization independently53. We found that Sirt3 has a strong correlation with ScvO2, but this correlation remains limited to the septic shock group, which reflects poorer cellular oxygen utilization during septic shock. Mitochondria are the main site for the utilization of oxygen, we discovered that there was a significant difference in ScvO2 between the low Sirt3 and high Sirt3 groups when the factors that influence ScvO2 did not differ statistically, but this result did not appear between the sepsis group and septic shock groups, which implied that Sirt3 had the potential to be an indicator of cellular oxygen utilization in response to septic shock.
The massive release of inflammatory factors is closely related to mitochondrial metabolism. During sepsis, immune cells switch to glycolysis, numerous metabolites (like lactate, citrate, and succinate), produced by glycolysis and fractured TCA, accumulate in the cell to facilitate signaling and epigenetic interactions which drive the inflammatory response and secrete a large number of inflammatory factors (TNF-α, IL-6)54. Sirt3 is the bridge between mitochondrial metabolism and immune cell activity, and downregulation of Sirt3 reprograms mitochondrial metabolism and promotes macrophage death55. Sirt3 can also affect immune cell survival by regulating mitochondrial homeostasis56. Our results showed that the lower the level of Sirt3, the worse the indicators of inflammation (TNF-α, IL-6) and infection (PCT), a result that was expected.
Mitochondria's role in diseases is no longer limited to provide energy to cells, they also play a role in signaling, gene control, cellular calcium regulation, and activation of cell death pathways. Sirt3 influences mitochondrial intracellular function through the homeostatic regulation of mitochondrial fission57, biosynthesis58, and autophagy59, in addition to altering their metabolic activity. The results of this study predicted that patients with lower serum Sirt3 levels have a worse prognosis, and our survival analysis backed up this prediction. The clinical significance of Sirt3 in sepsis was confirmed by our study.
Limitation
Despite the findings, there are still many limitations. In our study, we didn't find a stronger correlation between Sirt3 and the sepsis group, and we also didn't find a correlation between lactate and Sirt3, as well as we failed to use Sirt3 to identify patients at risk of developing septic shock from sepsis, which could be due to the limited sample size. This is a single-center study, so generalizability may be limited, and it's also unclear whether different treatments influenced the findings. In the future, we hope for bigger multicenter follow-up cohort studies to look into the role of Sirt3 in sepsis.
Conclusion
Sirt3 is a promising biomarker for the diagnosis of sepsis and predicting mortality risk in septic patients. Low serum levels of Sirt3 are significantly correlated with the disease severity. In septic shock, Sirt3 can bridge the gap in the specific response of lactate to the "cytopathic hypoxia". Patients with low levels of Sirt3 have a worse prognosis and lower 28-day survival.
Acknowledgements
We thank all patients for their understanding and cooperation during this study and their contributions to sepsis research.
Author contributions
J.J.L. and D.W.L. discussed and performed the study. G.S.Z. and H.M.Z. were involved in the diagnosis and screening of patients. G.S.Z., R.P.C. and Z.W.T. collected serum samples and clinical information from patients. J.J.L. and G.S.Z. analyzed data and drew graphs. J.J.L. wrote the main manuscript, X.T.W. and D.W.L. revised the manuscript and coordinated the work.
Funding
Thanks to the Beijing Municipal Science & Technology Commission for the financial support (No. Z201100005520038).
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro NI, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 2005;45:524–528. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Nichol A, et al. Dynamic lactate indices as predictors of outcome in critically ill patients. Crit. Care. 2011;15:242. doi: 10.1186/cc10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piñeros Alvarez AR, et al. SOCS1 is a negative regulator of metabolic reprogramming during sepsis. JCI Insight. 2017;2:2. doi: 10.1172/jci.insight.92530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Feng YW, Yao YM. Potential therapy strategy: Targeting mitochondrial dysfunction in sepsis. Mil. Med. Res. 2018;5:41. doi: 10.18502/rmm.v5i4.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacha GL, et al. Association of catecholamine dose, lactate, and shock duration at vasopressin initiation with mortality in patients with septic shock. Crit. Care Med. 2021;2:2. doi: 10.1097/CCM.0000000000005317. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit. Care. 2014;18:503. doi: 10.1186/s13054-014-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet (Lond., Engl.) 2004;364:545–548. doi: 10.1016/S0140-6736(04)16815-3. [DOI] [PubMed] [Google Scholar]
- 9.Fink MP. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit. Care Clin. 2001;17:219–237. doi: 10.1016/S0749-0704(05)70161-5. [DOI] [PubMed] [Google Scholar]
- 10.Hoeyer-Nielsen AK, et al. Association between the oxygen consumption: Lactate ratio and survival in critically ill patients with sepsis. Shock. 2021;55:775–781. doi: 10.1097/SHK.0000000000001661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zapelini PH, et al. Antioxidant treatment reverses mitochondrial dysfunction in a sepsis animal model. Mitochondrion. 2008;8:211–218. doi: 10.1016/j.mito.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Mantzarlis K, Tsolaki V, Zakynthinos E. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid. Med. Cell. Longev. 2017;2017:5985209. doi: 10.1155/2017/5985209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koentges C, et al. Impaired SIRT3 activity mediates cardiac dysfunction in endotoxemia by calpain-dependent disruption of ATP synthesis. J. Mol. Cell. Cardiol. 2019;133:138–147. doi: 10.1016/j.yjmcc.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Koos B, et al. LPS-induced endotoxemia evokes epigenetic alterations in mitochondrial DNA that impacts inflammatory response. Cells. 2020;9:2. doi: 10.3390/cells9102282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domínguez-Andrés J, et al. The itaconate pathway is a central regulatory node linking innate immune tolerance and trained immunity. Cell. Metab. 2019;29:211–220. doi: 10.1016/j.cmet.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5:66–72. doi: 10.4161/viru.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Wyngene L, Vandewalle J, Libert C. Reprogramming of basic metabolic pathways in microbial sepsis: therapeutic targets at last? EMBO Mol. Med. 2018;10:2. doi: 10.15252/emmm.201708712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorente L, et al. Platelet cytochrome c oxidase activity and quantity in septic patients. Crit. Care Med. 2011;39:1289–1294. doi: 10.1097/CCM.0b013e31820ee20c. [DOI] [PubMed] [Google Scholar]
- 19.Zeng Z, et al. The pyruvate dehydrogenase complex in sepsis: metabolic regulation and targeted therapy. Front. Nutr. 2021;8:783164. doi: 10.3389/fnut.2021.783164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCall CE, et al. Pyruvate dehydrogenase complex stimulation promotes immunometabolic homeostasis and sepsis survival. JCI insight. 2018;3:2. doi: 10.1172/jci.insight.99292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preau S, et al. Energetic dysfunction in sepsis: a narrative review. Ann. Intensive Care. 2021;11:104. doi: 10.1186/s13613-021-00893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brealey D, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet (London, England) 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 23.Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: Energy production, apoptosis, and signaling. Trends Biochem. Sci. 2010;35:669–675. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finley LW, et al. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS ONE. 2011;6:e23295. doi: 10.1371/journal.pone.0023295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirschey MD, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wellen KE, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He J, et al. Glucose limitation activates AMPK coupled SENP1-Sirt3 signalling in mitochondria for T cell memory development. Nat. Commun. 2021;12:4371. doi: 10.1038/s41467-021-24619-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrico C, Meyer JG, He W, Gibson BW, Verdin E. The mitochondrial acylome emerges: Proteomics, regulation by sirtuins, and metabolic and disease implications. Cell Metab. 2018;27:497–512. doi: 10.1016/j.cmet.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, et al. Sirt3 is a novel target to treat sepsis induced myocardial dysfunction by acetylated modulation of critical enzymes within cardiac tricarboxylic acid cycle. Pharmacol. Res. 2020;159:104887. doi: 10.1016/j.phrs.2020.104887. [DOI] [PubMed] [Google Scholar]
- 30.Jin K, et al. Activation of AMP-activated protein kinase during sepsis/inflammation improves survival by preserving cellular metabolic fitness. FASEB J. 2020;34:7036–7057. doi: 10.1096/fj.201901900R. [DOI] [PubMed] [Google Scholar]
- 31.Yu W, et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:1973–1983. doi: 10.1016/j.bbadis.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Ansari A, et al. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell. 2017;16:4–16. doi: 10.1111/acel.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar R, et al. Identification of serum sirtuins as novel noninvasive protein markers for frailty. Aging Cell. 2014;13:975–980. doi: 10.1111/acel.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Cheng S, Chen S, Zhao Y. Prognostic and clinicopathological value of SIRT3 expression in various cancers: A systematic review and meta-analysis. Onco. Targets. Ther. 2018;11:2157–2167. doi: 10.2147/OTT.S157836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang GC, et al. The expression and related clinical significance of SIRT3 in non-small-cell lung cancer. Dis. Markers. 2017;2017:8241953. doi: 10.1155/2017/8241953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu H, et al. SIRT3-AMPK signaling pathway as a protective target in endothelial dysfunction of early sepsis. Int. Immunopharmacol. 2022;106:108600. doi: 10.1016/j.intimp.2022.108600. [DOI] [PubMed] [Google Scholar]
- 37.Yang X, et al. Tubeimoside I improves endothelial function in sepsis via activation of SIRT3. Lab. Investig. 2021;101:897–907. doi: 10.1038/s41374-021-00580-y. [DOI] [PubMed] [Google Scholar]
- 38.Cheng Z, et al. Tubeimoside I protects against sepsis-induced cardiac dysfunction via SIRT3. Eur. J. Pharmacol. 2021;905:174186. doi: 10.1016/j.ejphar.2021.174186. [DOI] [PubMed] [Google Scholar]
- 39.Xin T, Lu C. SirT3 activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury. Aging. 2020;12:16224–16237. doi: 10.18632/aging.103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan C, et al. Inhibition of aerobic glycolysis alleviates sepsis-induced acute kidney injury by promoting lactate/Sirtuin 3/AMPK-regulated autophagy. Int. J. Mol. Med. 2021;47:2. doi: 10.3892/ijmm.2021.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu S, et al. Melatonin attenuates sepsis-induced small-intestine injury by upregulating SIRT3-mediated oxidative-stress inhibition, mitochondrial protection, and autophagy induction. Front. Immunol. 2021;12:625627. doi: 10.3389/fimmu.2021.625627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, et al. Sirtuins and immuno-metabolism of sepsis. Int. J. Mol. Sci. 2018;19:2. doi: 10.3390/ijms19092738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhai M, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J. Pineal Res. 2017;63:2. doi: 10.1111/jpi.12419. [DOI] [PubMed] [Google Scholar]
- 44.Wang T, et al. SENP1-Sirt3 signaling controls mitochondrial protein acetylation and metabolism. Mol. Cell. 2019;75:823–834. doi: 10.1016/j.molcel.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Bi S, et al. SIRT7 antagonizes human stem cell aging as a heterochromatin stabilizer. Protein Cell. 2020;11:483–504. doi: 10.1007/s13238-020-00728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao WY, Zhang L, Sui MX, Zhu YH, Zeng L. Protective effects of sirtuin 3 in a murine model of sepsis-induced acute kidney injury. Sci. Rep. 2016;6:33201. doi: 10.1038/srep33201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haden DW, et al. Mitochondrial biogenesis restores oxidative metabolism during Staphylococcus aureus sepsis. Am. J. Respir. Crit. Care Med. 2007;176:768–777. doi: 10.1164/rccm.200701-161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy RJ, et al. Evidence of myocardial hibernation in the septic heart. Crit. Care Med. 2005;33:2752–2756. doi: 10.1097/01.CCM.0000189943.60945.77. [DOI] [PubMed] [Google Scholar]
- 49.Hernández G, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: The ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;321:654–664. doi: 10.1001/jama.2019.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mok G, et al. Macrocirculatory and microcirculatory endpoints in sepsis resuscitation. J. Intensive Care Med. 2021;36:1385–1391. doi: 10.1177/0885066620982585. [DOI] [PubMed] [Google Scholar]
- 51.Alamdari N, et al. Temporal changes in the involvement of pyruvate dehydrogenase complex in muscle lactate accumulation during lipopolysaccharide infusion in rats. J. Physiol. 2008;586:1767–1775. doi: 10.1113/jphysiol.2007.149625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vincent JL, Bakker J. Blood lactate levels in sepsis: in 8 questions. Curr. Opin. Crit. Care. 2021;27:298–302. doi: 10.1097/MCC.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 53.Ince C, Mik EG. Microcirculatory and mitochondrial hypoxia in sepsis, shock, and resuscitation. J. Appl. Physiol. 2016;120:226–235. doi: 10.1152/japplphysiol.00298.2015. [DOI] [PubMed] [Google Scholar]
- 54.Soto-Heredero G, Gómez MM, de Las Heras E, Gabandé-Rodríguez JO, Mittelbrunn M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020;287:3350–3369. doi: 10.1111/febs.15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smulan LJ, et al. Sirtuin 3 downregulation in mycobacterium tuberculosis-infected macrophages reprograms mitochondrial metabolism and promotes cell death. MBio. 2021;12:2. doi: 10.1128/mBio.03140-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim YJ, et al. Sirtuin 3 is essential for host defense against Mycobacterium abscessus infection through regulation of mitochondrial homeostasis. Virulence. 2020;11:1225–1239. doi: 10.1080/21505594.2020.1809961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao W, et al. SIRT3 protects kidneys from ischemia-reperfusion injury by modulating the DRP1 pathway to induce mitochondrial autophagy. Life Sci. 2021;286:120005. doi: 10.1016/j.lfs.2021.120005. [DOI] [PubMed] [Google Scholar]
- 58.Ding Y, et al. Sirtuin 3 is required for osteogenic differentiation through maintenance of PGC-1ɑ-SOD2-mediated regulation of mitochondrial function. Int. J. Biol. Sci. 2017;13:254–264. doi: 10.7150/ijbs.17053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim TS, et al. SIRT3 promotes antimycobacterial defenses by coordinating mitochondrial and autophagic functions. Autophagy. 2019;15:1356–1375. doi: 10.1080/15548627.2019.1582743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.