Table 1.
Anti-protozoal activity and toxicity of pyridazines and tetrahydrophthalazinones.
| Cmpd | R1 | R2 | Y | M.W. | cLogPa | tPSAa | IC50 (nM) |
|
|---|---|---|---|---|---|---|---|---|
| G.l.b | MRC-5c | |||||||
| 11 | NO2 | MeO | Y1 | 531.6 | 3.3 | 127.7 | 0.5 ± 0.2 | >6.4 × 104 |
| 12 | NO2 | cyclopentyloxy | Y2 | 585.7 | 4.6 | 127.7 | 9.3 ± 0.6 | >6.4 × 104 |
| 13 | NO2 | 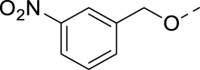 |
Y2 | 652.7 | 5.0 | 170.8 | 1.2 ± 0.4 | >6.4 × 104 |
| 14 | NO2 |  |
Y2 | 637.7 | 4.9 | 136.9 | 1.0 ± 0.5 | >6.4 × 104 |
| 15 | NO2 | 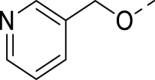 |
Y2 | 608.7 | 3.8 | 140.6 | 6.8 ± 1.5 | 15,000 ± 11,000 |
| 16 | NO2 | 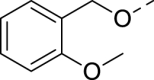 |
Y2 | 637.7 | 4.9 | 136.9 | 2.8 ± 0.8 | 49,000 ± 21,000 |
| 17 | NO2 |  |
Y2 | 608.7 | 3.9 | 140.6 | 0.5 ± 0.1 | >6.4 × 104 |
| 18 | NO2 | MeO | Y3 | 479.5 | 2.1 | 127.7 | 0.2 ± 0.1 | >6.4 × 104 |
| 19 | NO2 | Cl | Y3 | 483.9 | 2.9 | 118.5 | 19.0 ± 3.2 | >6.4 × 104 |
| 20 | H | MeO | Y3 | 434.5 | 2.1 | 84.5 | 930 ± 100 | >6.4 × 104 |
| 3 (MET) | - | - | - | 171.2 | −0.5 | 81.2 | 800 ± 100 | - |
| Tamoxifen | - | - | - | 371.5 | 6.8 | 12.5 | - | 11,000 ± 5,000 |
MET: metronidazole; G.l.: Giardia lamblia.
cLogP, tPSA are calculated using Collaborative Drug Discovery (CDD) Vault.
mean values ± standard errors are given for quadruplicates, the standard errors correspond to the 95% confidence intervals as calculated via the logit-log algorithm.
mean values ± standard deviations, n ≥ 2.

