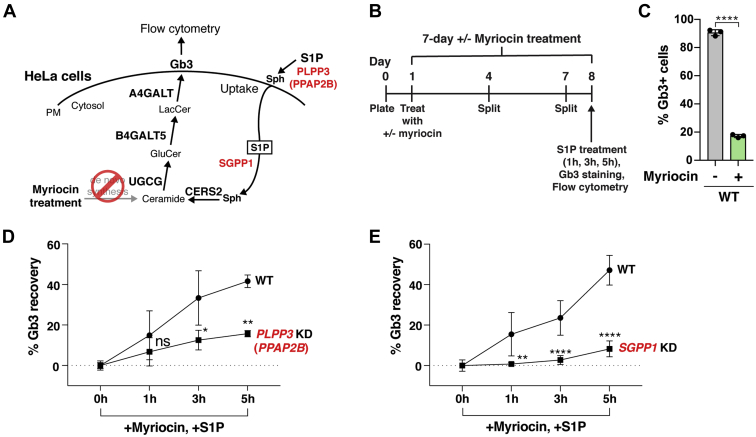
Fig. 3.
PLPP3 or SGPP1 disruption reduces S1P-stimulated Gb3 cell-surface expression. A: Schematic of the potential roles of PLPP3 (PPAP2B) and SGPP1 in the S1P uptake and recycling pathway. B: Timeline of experiment used to measure Gb3 recovery after 2.5 μM myriocin and 3 μM S1P treatment in KD HeLa cells and WT HeLa cells. C: Validation of myriocin inhibition of de novo Gb3 synthesis and subsequent cell-surface expression in WT HeLa cells. WT HeLa cells were cultured with 2.5 μM myriocin for 7 days and cell-surface Gb3 was measured by flow cytometry. Data represent the mean ± SD (n = 3). D: Gb3 cell-surface expression recovery rate after supplemental S1P treatment in myriocin-treated PLPP3 KD HeLa cells and WT HeLa cells, based on flow cytometry. Experiments were performed twice using one PLPP3 KD clone and WT HeLa cells (n = 6). E: Gb3 cell-surface expression recovery rate after supplemental S1P treatment in myriocin-treated SGPP1 KD HeLa cells and WT HeLa cells, based on flow cytometry. Experiments were performed three times using two SGPP1 KD clones and WT HeLa cells (n = 9 for WT, n = 12 for SGPP1). D and E: Gb3 cell-surface expression in the cells treated only with myriocin (0 h of S1P supplementation) was used as a baseline and subtracted from each data value. Experiments were conducted in triplicate wells for each condition. Data are presented as mean ± SD. P values were determined by unpaired t-tests; ∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < 0.0001. ns, not significant. Gb3, globotriaosylceramide; KD, knockdown; PM, plasma membrane; Sph, sphingosine, S1P, sphingosine-1-phosphate.