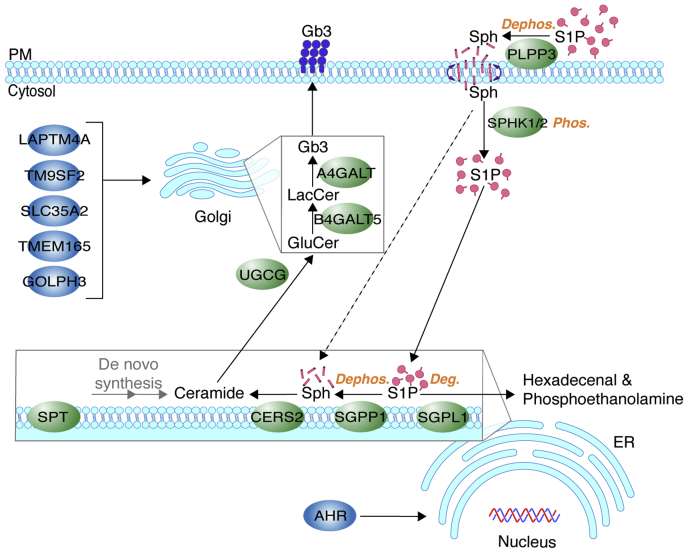
Fig. 7.
Proposed pathway for S1P cellular uptake and incorporation into the sphingolipid synthesis pathway. Plasma membrane–resident PLPP3 dephosphorylates exogenous S1P to sphingosine, which enters cells by a flip-flop mechanism and is phosphorylated to S1P by SPHKs 1 and 2. Intracellular S1P serves as a branch-point substrate for ER-resident SGPP1 and SGPL1. SGPP1 dephosphorylates S1P to sphingosine, which is utilized for ceramide synthesis (CERS2) and sequential modifications for glycosphingolipid production. SGPL1 irreversibly degrades S1P to hexadecenal and phosphoethanolamine, allowing exit of the substrate from the sphingolipid synthesis pathway. These enzymes work in concert to drive an S1P dephosphorylation-phosphorylation-dephosphorylation/degradation cycle. In the absence of sphingosine kinases, sphingosine may bypass the cycle and directly serve as a CERS2 substrate (dashed line), thereby being excluded from the SGPL1-mediated degradation pathway. UGCG, B4GALT5, and A4GALT are Golgi core glycosphingolipid synthesis enzymes for synthesis of Gb3. LAPTM4A, TM9SF2, SLC35A2, TMEM165, and GOLPH3 are positive regulators of Gb3 synthesis in the Golgi. AHR is a transcriptional activator of genes involved in glycosphingolipid synthesis. Deg, degradation; Dephos, dephosphorylation; Gb3, globotriaosylceramide; Phos, phosphorylation; PM, plasma membrane; Sph, sphingosine; S1P, sphingosine-1-phosphate