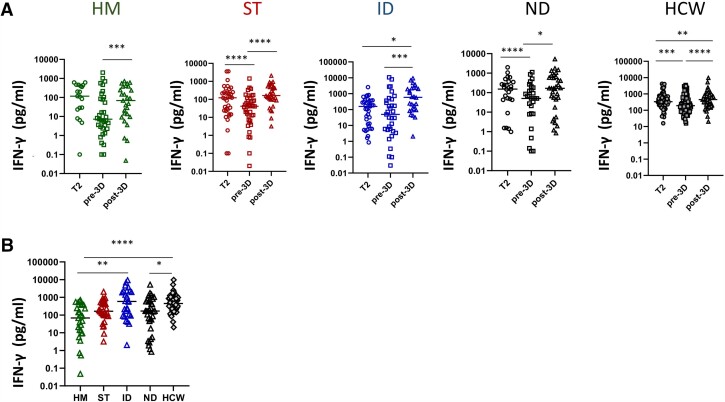
Figure 5.
Kinetics of T-cell response after 2 and 3 doses of vaccine in diseases groups. A, S-specific T-cell response expressed as pg/mL of IFN-γ was quantified before and after the booster dose in HM, ST, ID, ND and HCW. Differences were evaluated by Wilcoxon paired test. HM: median T2: 116.5 pg/mL (IQR 23.2–436.6 pg/mL); median pre-3D: 7.24 pg/mL (IQR 2.3–94.6 pg/mL); median post-3D: 68.5 pg/mL (IQR 9.5–378.3 pg/mL). *** P < .001. ST: median T2: 122.8 pg/mL (IQR 23.0–250.8 pg/mL); median pre-3D: 41.3 pg/mL (IQR 10.0–104.7 pg/mL); median post-3D: 163.1 pg/mL (IQR 95.2–522.7 pg/mL). ****P < .0001. ID: median T2: 158.45 pg/mL (IQR 10.6–354.8 pg/mL); median pre-3D: 49.4 pg/mL (IQR 4.8–402.3 pg/mL); median post-3D: 590.2 pg/mL (IQR 92.4–2223.0 pg/mL). *P < .05, ***P < .001. ND: median T2: 152.8 pg/mL (IQR 42.9–358.6 pg/mL); median pre-3D: 50.6 pg/mL (IQR 8.3-170.3 pg/mL); median post-3D: 167.5 pg/ml (IQR 31.6-624.5 pg/mL). *P < .05, ***P < .001. HCW: median T2: 335.9 pg/mL (IQR 199.0–679.0 pg/mL); median pre-3D: 190.8 pg/mL (IQR 88.7–437.2 pg/ml); median post-3D: 448.9 pg/mL (IQR 197.3–862.2 pg/mL). **P < .01, ***P < .001, ****P < .0001. B, S-specific T-cell response at Tpost-3D was compared among groups and was expressed as pg/mL. Differences were evaluated by Kruskal-Wallis test. *P < .05; **P < .01; ****P < .0001. HM: median = 68.6 pg/mL (IQR 9.5–378.3 pg/mL); ST: 163.1 pg/mL (IQR 95.2–522.7 pg/mL); ID: 590.2 pg/mL (IQR 92.4–2223.0 pg/mL); ND: 167.5 pg/mL (IQR 31.6–624.5 pg/mL) and HCW: 448.9 pg/mL (IQR 197.3–852.2 pg/mL). Abbreviations: HCW, health care workers; HM, hematological malignancies; ID, immune-rheumatological diseases; IQR. interquartile range; ND, neurological disorders; ST, solid tumors; T2, 5–8 weeks from T0; Tpre-3D, 5 months from T2; Tpost-3D, 2–4 weeks from Tpre-3D.