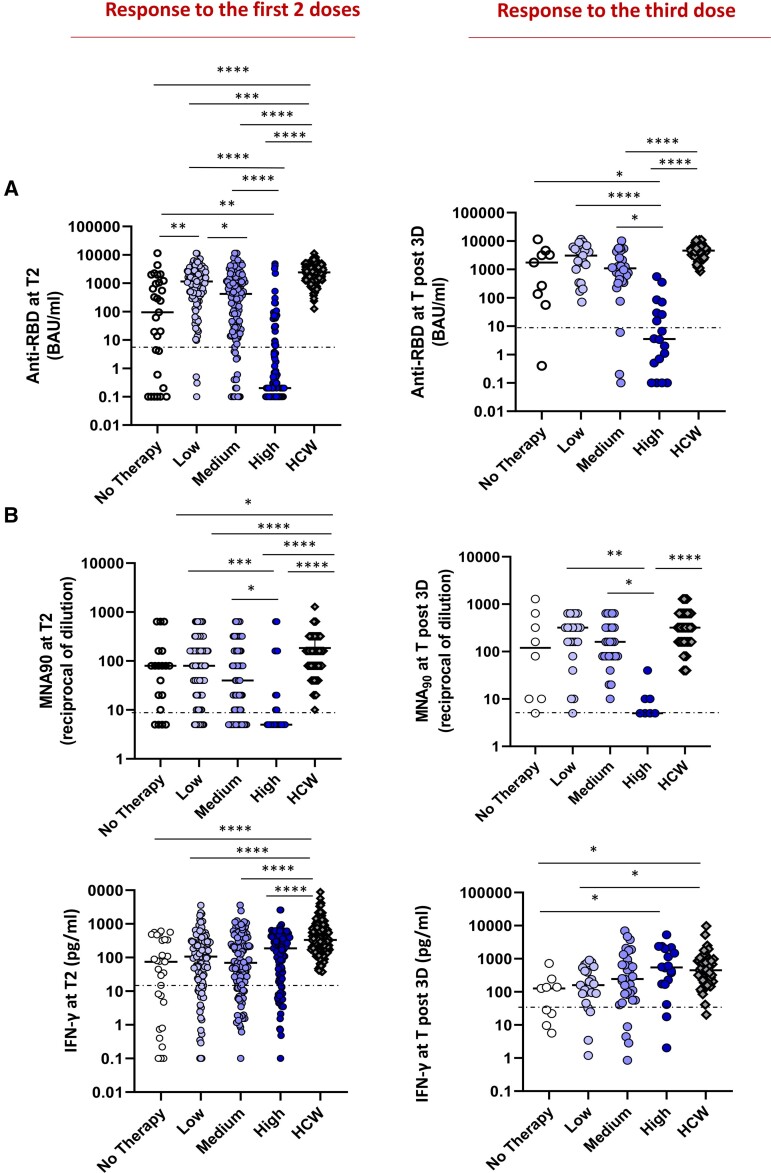
Figure 6.
Impact of different therapy on immune response. Independently from the diseases, the patients were divided on the basis of therapy in 4 groups: untreated (white dots) or treated by therapy with a low (light violet dots), medium (dark violet dots), and high (blue dots) impact on the immune system. A group of HCW was added as a control. The immunogenicity of 2 or 3 doses of vaccine was compared among groups. A, The levels of anti-RBD in the 4 patient groups and in HCW are shown. Differences were evaluated by Kruskal-Wallis test. **P < .01; ***P < .001; ****P < .0001. Response after 2 doses: no therapy: median = 94.2 BAU/mL (IQR .4–1133.0 BAU/mL); low impact: 1144.0 BAU/mL (IQR 368.1–11,360.0 BAU/mL); medium impact: 420.3 BAU/mL (IQR 17.4–1563.0 BAU/mL); high impact: 0.2 (IQR 0.1–6.4 BAU/mL); HCW: 2405.0 BAU/mL (IQR 1343–3848 BAU/mL). Response after the third dose: no therapy: median = 1748 BAU/mL (IQR 95.4–3917.0 BAU/mL); low impact: 3044 BAU/mL (IQR 998.8–6175.0 BAU/mL); medium impact: 1088.0 BAU/mL (IQR 380.1–2536.0 BAU/mL); high impact: 3.5 (IQR .4–39.6 BAU/mL); HCW: post-3D: 4608.0 BAU/mL (IQR 3302.0–6030.0 BAU/mL). B, The level of neutralizing antibodies in the 4 groups is shown. Differences were evaluated by Kruskal-Wallis test. *P < .05; ****P < .0001. Response after 2 doses: no therapy: median = 80 reciprocal of dilution (IQR 10–160); low impact: 80 reciprocal of dilution (IQR 20–160); medium impact: 40 reciprocal of dilution (IQR 6.2–160.0); high impact: 5 reciprocal of dilution (IQR 5–20); HCW: 160 (IQR 80–320). Response after the third dose: no therapy: median = 120 reciprocal of dilution (IQR 10–560); low impact: 320 reciprocal of dilution (IQR 140–400); medium impact: 160 reciprocal of dilution (IQR 80–320); high impact: 5 reciprocal of dilution (IQR 5–10); HCW: 320 reciprocal of dilution (IQR 160–640). C: The T-cell response, analyzed by quantifying IFN-γ in the 4 groups is shown. Differences were evaluated by Kruskal-Wallis test. Response after 2 doses: no therapy: median = 74.9 pg/mL (IQR 2.7–338.4 pg/mL); low impact: 105.9 pg/mL (IQR 26.3–291.9 pg/mL); medium impact: 69.2 pg/mL (IQR 18.1–297.3 pg/mL); high impact: 186.3 pg/mL (IQR 22.7–390.0); HCW: 331.9 pg/mL (IQR 189.9–765.0 pg/mL). Response after the third dose: no therapy: median = 127.2 pg/mL (IQR 15.8–192.0 pg/mL); low impact: 158.9 pg/mL (IQR 87.2–536.4 pg/mL); medium impact: 243.4 pg/mL (IQR 69.3–799.7 pg/mL); high impact: 545.3 pg/mL (IQR 171.9–2049.0). HCW: 448.9 pg/mL (IQR 197.3–852.2 pg/mL). Abbreviations: HCW, health care workers; IQR, interquartile range; RBD, receptor binding domain; T2, 5–8 weeks from T0; Tpost-3D, 2–4 weeks from Tpre-3D.