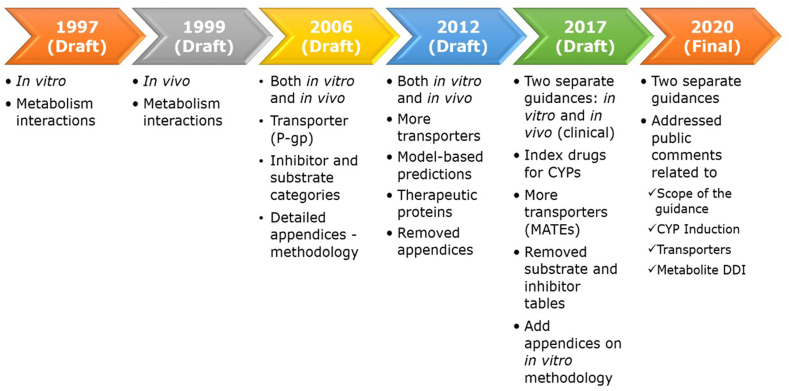
Figure 3.
FDA DDI Guidance History (1997–2020). Major CYP enzymes recommended for routine assessment to identify potential metabolism-mediated interactions include: CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A. Transporter-mediated DDI was first recommended in 2006 draft FDA DDI guidance, focusing on P-glycoprotein. Evolving knowledge on roles of transporters in DDI, safety and efficacy, and collaborative work led by the International Transporter Consortium has led to more transporters being studied105,276, 277, 278. To date, nine clinically important transporters are recommended in FDA DDI guidance documents for routine evaluations of DDI potential for investigational drugs, which include four efflux transporters (P-gp, BCRP, MATE1 and MATE2-K) and five uptake transporters (OATP1B1, OATP1B3, OAT1, OAT3, and OCT2)279,280. Basic and more mechanistic models and decision criteria have been developed and refined over the past decade. The international harmonization efforts on DDI evaluation are being pursued at the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). An ICH M12 guideline on DDI is being developed to provide a consistent approach in designing, conducting, and interpreting DDI studies during the development of a therapeutic product (https://www.ich.org/page/multidisciplinary-guidelines).