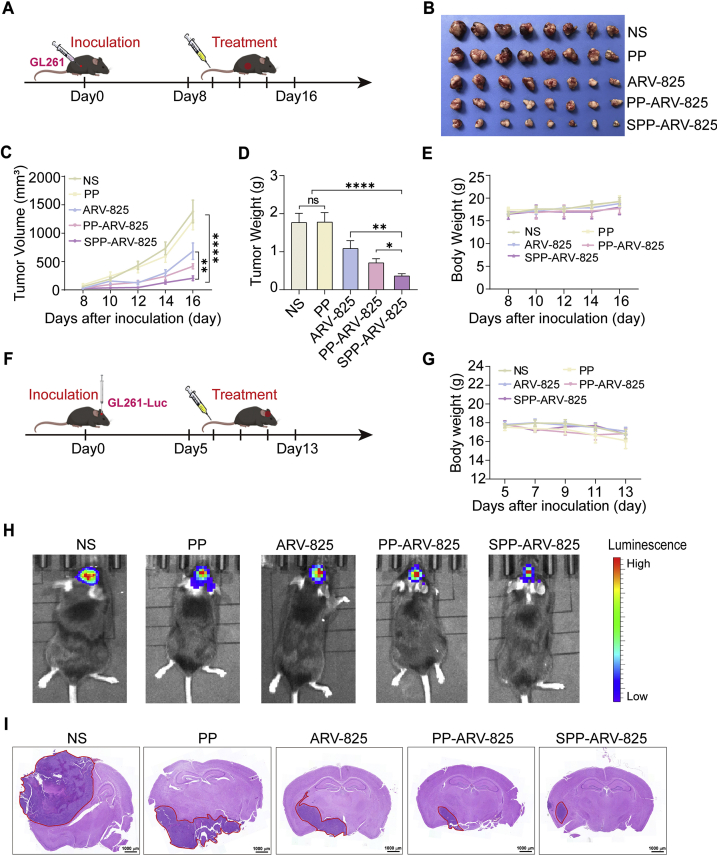
Figure 4.
Antitumor effect of SPP-ARV-825 in GL261 glioma subcutaneous model and orthotopic model. (A) Schedule of experimental design in mouse subcutaneous model. (B) The representative image of tumors excised from mice treated with NS, PP, ARV-825, PP-ARV-825 and SPP-ARV-825 at ARV-825 dose of 20 mg/kg, respectively. (C) Tumor growth curves for GL261 tumor-bearing mice treated with different formulation (n = 8). (D) Tumor weights of mice receiving different treatments (n = 8). (E) Changes of body weight of mice in different group during treatment (n = 8). (F) Schedule of experimental design in mouse orthotopic model. (G) Body weight changes of mice with orthotopic tumor during treatment (n = 6). (H) In vivo imaging of GL261-Luc tumor-bearing mice at end of different treatments. (I) Representative H&E staining images of brain tissues from different therapeutic groups (scale bar = 1000 μm). Red circles in images show tumors. Data are presented as mean ± SEM. No significant difference is marked with ns. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗∗P < 0.0001.