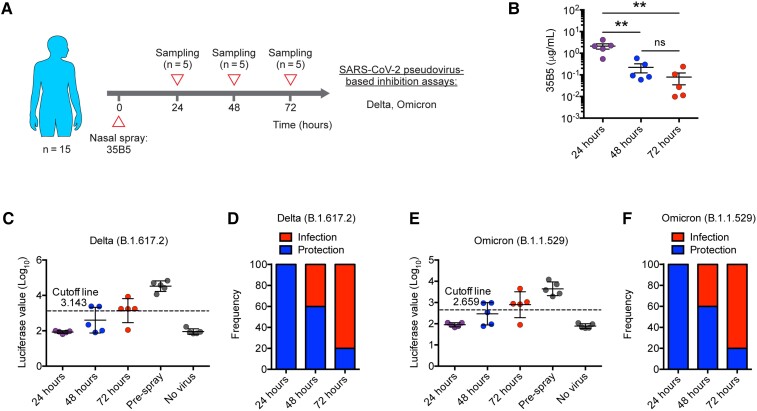
Figure 2.
Protection efficacy of a single dose of 35B5 nasal spray wanes after 24 hours. A, Schematic diagram. B, 35B5 monoclonal antibody concentrations in nasal mucosal samples at different timepoints as determined by enzyme-linked immunosorbent assay. C–F, Neutralization of nasal mucosal samples against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta (C and D) and Omicron (E and F) variants as determined by pseudovirus neutralization assays. Nasal secretions from individuals with luciferase value under the cutoff line were considered protective. The cutoff value in each pseudovirus-neutralizing function assay was determined by receiver operating characteristic curve analysis and was of the highest likelihood ratio to distinguish samples of positive controls (pre–antibody spray) and samples of negative controls (no virus). Error bars in B indicate standard error of the mean, and error bars in C and E indicate standard deviation. **P < .01. Abbreviation: ns, not significant.