Abstract
Background
Single-dose vaccination was widely recommended in the pre-Omicron era for persons with previous SARS-CoV-2 infection. The effectiveness of a second vaccine dose in this group in the Omicron era is unknown.
Methods
We linked nationwide population registries in Spain to identify community-dwelling individuals aged 18–64, with a positive SARS-CoV-2 test before single-dose mRNA vaccination (mRNA-1273 or BNT162b2). Every day between 3 January and 6 February 2022 we matched 1:1 individuals receiving a second mRNA vaccine dose and controls on sex, age, province, first dose type and time, month of primary infection, and number of previous tests. We then estimated Kaplan–Meier risks of confirmed SARS-CoV-2 reinfection. We performed a similar analysis in a Delta-dominant period, between 19 July and 30 November 2021.
Results
In the Omicron period, estimated effectiveness (95% CI) of a second dose was 62.2% (58.2–66.4%) 7–34 days after administration, similar across groups defined by age, sex, type of first vaccine, and time since the first dose. Estimated effectiveness was 65.4% (61.1–69.9%) for mRNA-1273 and 52.0% (41.8–63.1%) for BNT162b2. Estimated effectiveness was 78.5% (67.4–89.9%), 66.1% (54.9–77.5%), and 60.2% (55.5–64.8%) when primary infection had occurred in the Delta, Alpha, and pre-Alpha periods, respectively. In the Delta period, the estimated effectiveness of a second dose was 8.8% (−55.3% to 81.1%).
Conclusions
Our results suggest that, over 1 month after administration, a second dose of mRNA vaccine increases protection against SARS-CoV-2 reinfection with the Omicron variant among individuals with single-dose vaccination and previously infected with another variant.
Keywords: COVID-19, SARS-CoV-2, Omicron, vaccines, effectiveness
A second vaccine dose increases protection against infection with the SARS-CoV-2 Omicron variant by 62% (58.2–66.4%) among individuals previously infected with a single-dose vaccination schedule. In the previous Delta-dominant period no benefit of a second vaccine dose was observed.
Adequate protection against infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta variant requires at least 2 doses of mRNA vaccines in persons not previously infected. However, it is unknown whether a second dose is necessary in individuals with prior infection. In fact, 13 countries in Europe, Spain among them, considered single-dose vaccination as complete vaccination for individuals with a previously confirmed SARS-CoV-2 infection [1–3].
The decision to withhold the second dose was supported by findings of high vaccine effectiveness and long-term preservation of the protection afforded by 1 vaccine dose in previously infected individuals [4–14]. However, these findings predated the emergence of the Omicron variant, which has an increased capacity to elude immunity [15–18]. Even if a second vaccine dose were unnecessary to protect previously infected individuals against reinfection with the Delta variant, a second dose may be necessary to provide adequate protection against reinfection with the Omicron variant. Several countries, including Spain, recommended a second dose to individuals with prior infection in the Omicron era as part of the population-wide booster campaign [2].
Here we estimate the effectiveness of a second dose of mRNA vaccine against confirmed SARS-CoV-2 reinfection, during a period of Omicron predominance, in individuals under 65 years of age with previous infection and who had received a single dose after the primary infection. We estimated the effectiveness among residents of Spain overall and by age, sex, calendar period of the primary infection, interval between the first and the second vaccine dose, type of vaccine used as first dose, and type of vaccine used as second dose.
METHODS
Study Population
We used a unique personal identifier to link individual-level data from the Vaccination Registry (REGVACU) and the Laboratory Results Registry (SERLAB), both updated daily and with nationwide coverage. SERLAB includes all SARS-CoV-2 polymerase chain reaction (PCR) and rapid antigen tests performed by healthcare providers and, since 21 December 2021, it also includes results from self-administered rapid antigen tests from certain regions. We subtracted 2 days from PCR test results to approximate the date of sample collection. To increase the probability that individuals were present in Spain during the study period, we restricted the study to individuals successfully matched in the National Health System registry, which virtually includes the full population accessing the healthcare system in Spain. We excluded individuals who were only temporarily entitled to access the health system.
Specification of the Target Trial
Our observational study emulated a target trial to estimate the effect of the administration of a second dose of BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccines for the prevention of reinfection with SARS-CoV-2 in a period of Omicron SARS-CoV-2 dominance among individuals who had a previous infection followed by just 1 dose of the vaccine. Recruitment was from 3 January 2022, when Omicron was more than 90% [19] of SARS-CoV-2–detected variants in Spain, to 6 February 2022.
Eligibility criteria were age between 18 and 64 years at first vaccine dose, laboratory-confirmed SARS-CoV-2 infection before first vaccine dose and before Omicron became more than 50% of circulating variants (27 December 2021), entitlement to access healthcare in Spain, vaccination with a first vaccine dose of mRNA-1273 or BNT162b2 at least 19 days ago and during the period recommended for their age group (to exclude essential workers, particularly in the education sector, immunosuppressed individuals, and others with different probability of infection than the general population), not a member of groups with specific vaccine recommendations (eg, nursing home residents, institutionalized individuals, healthcare workers), and no documented SARS-CoV-2 reinfection after first vaccine dose.
In the target trial, eligible persons would be randomly assigned to either administration of a second dose of an mRNA vaccine (mRNA-1273 or BNT162b2) or to no additional vaccine dose within strata defined by age, sex, province, time since first vaccination, time since previous infection, type of vaccine used as primary vaccination, and number of previous SARS-CoV-2 diagnostic tests. The outcome of interest was laboratory-confirmed SARS-CoV-2 infection.
Emulation of the Target Trial
We extracted the data on 14 February 2022. Each day between 3 January and 6 February 2022 we identified persons who met the eligibility criteria and classified them as either having or not having received a second dose on that day. Each person who received a second dose was matched to a randomly selected control (with replacement) on sex, age (5-year groups), province, type of vaccine used in the first dose, week of the first dose, month of the previous SARS-CoV-2 positive test, and total number of SARS-CoV-2 tests (both positive and negative) since the beginning of the pandemic (1, 2, ≥3). Eligible individuals could be selected as controls up to the day before the second dose. For each matched pair, follow-up started on the day of administration of the second dose and finished at the earliest of laboratory-confirmed SARS-CoV-2 infection, death or discontinuation of registration in the National Health System database, or 6 February 2022. We censored both members of a matched pair if/when the control received a second vaccine dose.
Statistical Analysis
We constructed cumulative incidence (risk) curves of laboratory-confirmed SARS-CoV-2 infection using the Kaplan–Meier estimator [20]. We compared the risks 7 or more days after the second vaccine dose via differences and ratios, and estimated effectiveness as 1 – risk ratio in all matched pairs in which both individuals were still at risk by day 7. We conducted analyses in the entire eligible population and in subgroups defined by age group, sex, type of vaccine used in the first dose, type of vaccine used in the second dose and time interval between the first and the second vaccine dose, and by calendar period when the primary infection occurred: pre-Alpha period (up to 7 February 2021), Alpha period (from 8 February to 4 July 2021), and Delta period (from 5 July to 26 December 2021).
For comparison purposes, we emulated a similar target trial, except that we included individuals who received the second vaccine dose between 19 July and 30 November 2021 (the period where Delta was ≥ 90% of all circulating variants) and their controls.
We carried out sensitivity analyses (1) restricted to persons with no test in the 7 days before time zero (to exclude contacts of cases or other persons exposed to SARS-CoV-2 who had tested early in their infection); in other sensitivity analyses, we (2) restricted to persons without any reported positive test in the previous 90 days, (3) used dates of laboratory tests as recorded (rather than subtracting 2 days for PCR tests), and (4) censored matched pairs 7 days after the control received a booster (rather than on the date of the booster).
We computed 95% confidence intervals (CIs) using nonparametric bootstrapping with 500 samples. Analyses were performed with R software version 4.1.2 (R Foundation for Statistical Computing).
This study was approved by the Research Ethics Committee at the Instituto de Salud Carlos III (approval no. CEI PI 98_2020 and CEI PI 08_2022).
RESULTS
Omicron Period
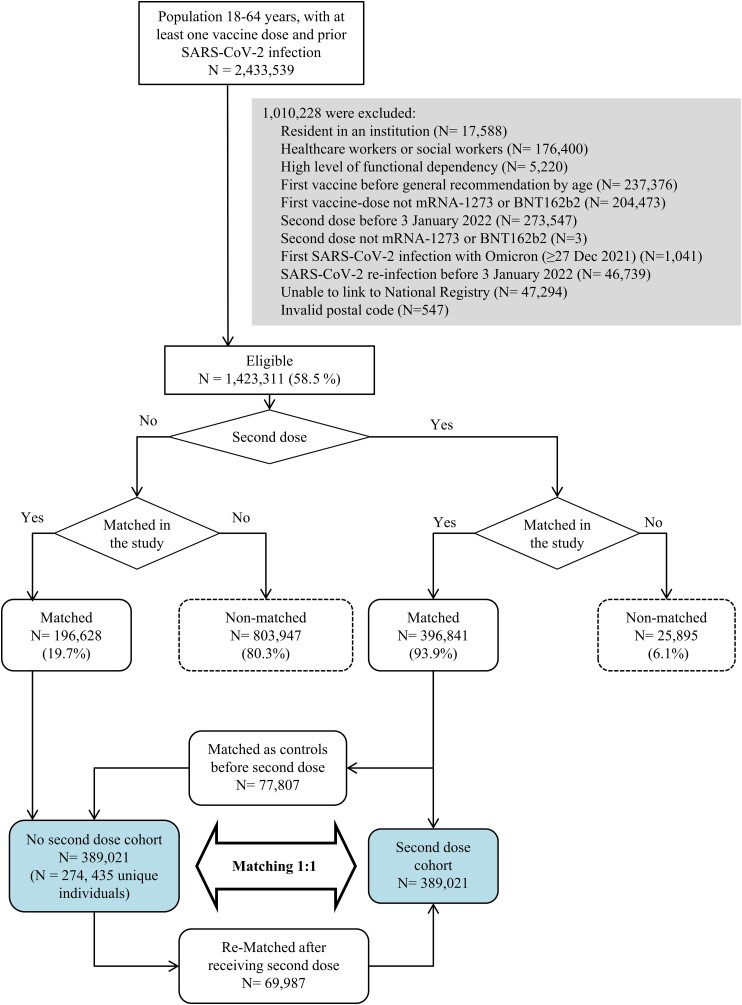
Of 2.4 million eligible individuals, 0.4 million received a second vaccine dose during the Omicron period, 69.2% with mRNA-1273 and 30.8% with BNT162b2 (Figure 1). We could match 389 021 individuals who received a second dose to the same number of controls who had not received the second dose up to that day (Table 1). Compared with the originally eligible population, the matched sample was slightly older, had used more frequently the BNT162b2 vaccine, both as the first and second dose, and corresponded more frequently to individuals first infected in the pre-Alpha period (Supplementary Table 1). The median age was 44 years.
Figure 1.
Sample selection flowchart. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Baseline Characteristics of the Matched Study Population, Spain: 3 January–6 February 2022
| Second Dose (n = 389 021) | No Second Dose (n = 389 021) | |
|---|---|---|
| Age (years) | ||
| ȃ18–24 | 39 634 (10.2%) | 40 289 (10.4%) |
| ȃ25–29 | 28 184 (7.2%) | 28 204 (7.2%) |
| ȃ30–34 | 37 831 (9.7%) | 37 156 (9.6%) |
| ȃ35–39 | 39 138 (10.1%) | 40 047 (10.3%) |
| ȃ40–44 | 61 104 (15.7%) | 61 286 (15.8%) |
| ȃ45–49 | 58 242 (15.0%) | 58 391 (15.0%) |
| ȃ50–54 | 67 199 (17.3%) | 66 497 (17.1%) |
| ȃ55–59 | 47 454 (12.2%) | 46 588 (12.0%) |
| ȃ60–64 | 10 235 (2.6%) | 10 563 (2.7%) |
| Sex | ||
| ȃFemale | 193 464 (49.7%) | 193 464 (49.7%) |
| ȃMale | 195 557 (50.3%) | 195 557 (50.3%) |
| Number of previous SARS-CoV-2 tests | ||
| ȃ1 | 129 127 (33.2%) | 129 127 (33.2%) |
| ȃ2 | 106 158 (27.3%) | 106 158 (27.3%) |
| ȃ≥3 | 153 736 (39.5%) | 153 736 (39.5%) |
| Type of vaccine used as first dose | ||
| ȃmRNA-1273 | 74 281 (19.1%) | 74 281 (19.1%) |
| ȃBNT162b2 | 314 740 (80.9%) | 314 740 (80.9%) |
| Type of vaccine used as second dose | ||
| ȃmRNA-1273 | 268 869 (69.1%) | … |
| ȃBNT162b2 | 120 152 (30.9%) | … |
| Time interval since vaccination with first dose | ||
| ȃ≤90 days | 5179 (1.3%) | 5179 (1.3%) |
| ȃ91–150 days | 26 136 (6.7%) | 26 136 (6.7%) |
| ȃ151–180 days | 109 646 (28.2%) | 109 646 (28.2%) |
| ȃ>180 days | 248 060 (63.8%) | 248 060 (63.8%) |
| Period of primary infection | ||
| ȃPre-Alpha | 296 929 (76.3%) | 297 372 (76.4%) |
| ȃAlpha | 73 239 (18.8%) | 72 923 (18.7%) |
| ȃDelta | 18 853 (4.8%) | 18 726 (4.8%) |
Data are presented as n (%). Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
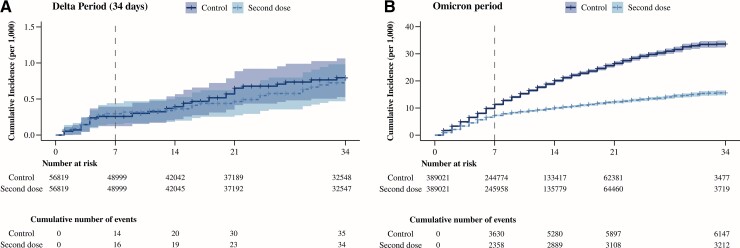
Maximum follow-up was 34 days, and the median (interquartile range [IQR]) follow-up was 10 (5–17) days; 32% of pairs were censored because the control individual received a second dose. During 9.1 million person-days, 3212 laboratory-confirmed SARS-CoV-2 infections occurred in the group who received the second dose and 6147 in the control group. The 34-day risk was 15.6 per 1000 in the second-dose group and 33.6 per 1000 in the control group (Figure 2). The number of tests per 1000 person-days 7 or more days after the booster (and before a coronavirus disease 2019 [COVID-19] diagnosis) was 2.0 in the group who received the second dose and 2.3 in the control group, of which less than 0.01% were recorded as self-tests in both groups. The respective positivity rates were 9.7% and 21.4% (Supplementary Figure 1).
Figure 2.
Estimates of COVID-19 risk by administration of a second vaccine dose, in individuals with infection prior to first vaccination, by period of administration of the second vaccine dose, Spain. (A) Delta period: 19 July–30 November 2021; (B) Omicron period: 3 January–6 February 2022. Abbreviation: COVID-19, coronavirus disease 2019.
The overall estimated effectiveness (95% CI) of the second dose was 62.2% (57.9–66.0%) at days 7–34 and 60.4% (53.8–66.7%) at days 14–34. Estimated effectiveness increased progressively to stabilize around day 7 (Supplementary Figure 2). The estimated number of cases averted at days 7–34 was 14.9 per 1000 individuals (13.4–16.4) (Figure 2, Table 2).
Table 2.
Estimated Effectiveness of a Second COVID-19 Vaccine Dose Against Laboratory-Confirmed SARS-CoV-2 Infection, Among Individuals Who Had Received a Single Vaccine Dose and Had a Laboratory-Confirmed Infection Before the First Vaccination, Spain: 3 January 3–6 February 2022
| Second Dose | No Second Dose | 1 – Risk Ratio (95% CI) | Risk Difference per 1000 (95% CI) | |||
|---|---|---|---|---|---|---|
| Events | Risk per 1000 | Events | Risk per 1000 | |||
| Overall | 999 | 9.1 | 2,858 | 24.0 | 62.2% (57.9–66.0%) | 14.9 (13.4–16.4) |
| Age (years) | ||||||
| ȃ18–24 | 72 | 7.6 | 190 | 22.8 | 66.9% (52.9–76.2%) | 15.3 (10.2–19.7) |
| ȃ25–49 | 645 | 11.1 | 1775 | 27.3 | 59.5% (53.5–64.9%) | 16.2 (14.0–18.8) |
| ȃ50–64 | 282 | 7.0 | 893 | 19.7 | 64.7% (57.1–71.3%) | 12.8 (10.8–15.0) |
| Sex | ||||||
| ȃFemale | 548 | 10.0 | 1594 | 26.7 | 62.6% (56.6–68.1%) | 16.7 (14.6–19.0) |
| ȃMale | 451 | 8.2 | 1264 | 21.4 | 61.7% (55.4–67.8%) | 13.2 (11.2–15.4) |
| Type of vaccine used as first dose | ||||||
| ȃmRNA-1273 | 158 | 7.6 | 399 | 19.6 | 61.0% (48.2–71.4%) | 11.9 (8.9–15.3) |
| ȃBNT162b2 | 841 | 9.4 | 2459 | 25.0 | 62.4% (57.8–66.5%) | 15.6 (13.8–17.2) |
| Type of vaccine used as second dose | ||||||
| ȃmRNA-1273 | 704 | 8.7 | 2179 | 25.1 | 65.4% (60.8–69.8%) | 16.4 (14.7–18.1) |
| ȃBNT162b2 | 295 | 10.2 | 679 | 21.3 | 52.0% (39.9–61.3%) | 11.1 (8.2–14.0) |
| Time interval since vaccination with first dose | ||||||
| ȃ≤90 days | 22 | 6.2 | 48 | 17.6 | 65.0% (41.9–81.0%) | 11.4 (5.3–18.0) |
| ȃ91–150 days | 64 | 8.4 | 184 | 24.0 | 65.1% (51.4–76.9%) | 15.6 (10.9–21.0) |
| ȃ151–180 days | 225 | 8.8 | 651 | 21.9 | 59.9% (48.4–68.8%) | 13.1 (9.8–16.2) |
| ȃ>180 days | 688 | 9.4 | 1975 | 24.6 | 61.6% (56.7–66.4%) | 15.2 (13.4–17.2) |
| Period of primary infection | ||||||
| ȃPre-Alpha | 854 | 9.7 | 2,354 | 24.5 | 60.2% (55.2–65.1%) | 14.7 (13.0–16.5) |
| ȃAlpha | 119 | 6.8 | 407 | 20.1 | 66.1% (52.7–75.8%) | 13.3 (9.7–16.9) |
| ȃDelta | 26 | 5.8 | 97 | 27.1 | 78.5% (64.4–87.0%) | 21.2 (14.0–28.4) |
Analyses based on 242 892 matched pairs who remained under follow-up by day 7 after the booster dose. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
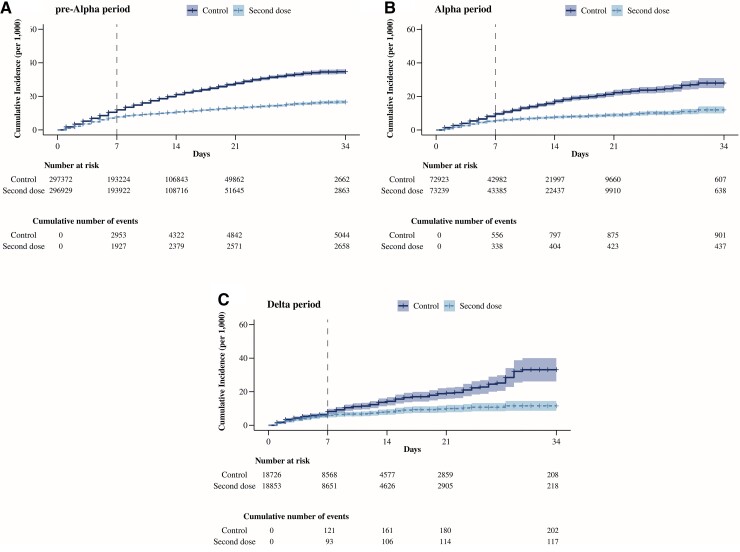
The estimated effectiveness (95% CI) was similar in subgroups defined by age, sex, type of vaccine used as first dose, or time elapsed since the first dose (Supplementary Figures 3–6, Table 2), but decreased with time since period of primary infection: 78.5% (64.4–87.0%) for individuals infected during the Delta period, 66.1% (52.7–75.8%) for the Alpha period, and 60.2% (55.2–65.1%) for the pre-Alpha period (Figure 3, Table 2). The estimated effectiveness of the second dose was higher for the mRNA-1273 vaccine (65.4%; 60.8–69.8%) than for the BNT162b2 vaccine (52.0%; 39.9–61.3%) (Supplementary Figure 7, Table 2). The effectiveness of a second dose of mRNA-1273 was 61.1% (47.9–71.4%) when the first dose had been mRNA-1273 and 66.3% (61.3–70.8%) when the first dose had been BNT162b2. The effectiveness of a second dose of BNT162b2 was 51.8% (39.8–61.2%) when the first dose had been BNT162b2, and 63.0% (29.4–83.8%) when the first dose had been mRNA-1273.
Figure 3.
Estimates of COVID-19 risk in individuals with SARS-CoV-2 infection before the first dose, by period of previous infection, Spain: 3 January to 6 February 2022. (A) Pre-Alpha period: up to 7 February 2021; (B) Alpha period: 8 February to 4 July 2021; (C) Delta period: 5 July–26 December 2021. Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In sensitivity analyses, the estimated effectiveness of an mRNA booster at days 7–34 in the Omicron era ranged between 59.8% and 64.1% (Supplementary Table 2).
Delta Period
We could match 56 819 individuals who received a second dose to the same number of controls who had not received the second dose up to that day (Supplementary Figure 8, Supplementary Table 3). The median age was 37 years and maximum follow-up was 133 days; the median (IQR) follow-up was 47 (13–98) days. During 6.4 million person-days, 65 laboratory-confirmed SARS-CoV-2 infections occurred in the group who received the second dose and 65 in the control group. In the first 34 days after study entry, during 2.8 million person-days, 34 and 35 laboratory-confirmed SARS-CoV-2 infections occurred, respectively.
The number of tests per 1000 person-days 7 or more days after the booster (and before a COVID-19 diagnosis) was 1.4 in the group who received the second dose and 0.9 in the control group. The positivity rates were 1.1% and 1.8%, respectively. In the first 34 days of follow up, the number of tests were, respectively, 2.0 and 3.1 per 1000 person-days, and positivity rates were 1.1% and 1.8%.
The 34-day risk was 0.80 per 1000 in both groups; the 133-day risk was 2.75 per 1000 in the group who received the second dose and 2.36 per 1000 in the control group (Figure 3). The estimated effectiveness (95% CI) was 8.8% (−79.8% to 54.2%) at days 7–34, −5% (−122.5% to 49.6%) at days 14–34, −16.9% (−93.1% to 33.8%) at days 7–133, and −21.3% (−109.9% to 28.2%) at days 14–133 (Supplementary Figure 9).
In sensitivity analyses, the estimated effectiveness of an mRNA booster at days 7–133 in the Delta period ranged between −35.1% and 28.6% (Supplementary Table 4) and at days 7–34 ranged between −50.3% and 12.1% (Supplementary Table 5).
DISCUSSION
In a nationwide follow-up study among individuals who had prior SARS-CoV-2 infection and had received a single-dose vaccine, we estimated that a second dose of an mRNA vaccine had an effectiveness of 62% during the Omicron period and of 8.8% during the Delta period, although this latter estimate was imprecise. These findings support current vaccination policies that recommend a second dose in the Omicron period, even if a second dose was not estimated to provide any benefit during the Delta period.
Our estimates are compatible with, but more precise than, those from a recent study in the Omicron era in people with previous infection [10], which also estimated a lower incidence of infection (hazard ratio: .77; 95% CI: .53–1.12) and symptomatic COVID-19 (hazard ratio: .36; 95% CI: .23–.57) among those who did versus those who did not receive a second dose. We did not find a benefit of administering a second vaccine dose to people with previous infection during the Delta period, which is consistent with previous findings of no benefit of a second dose in periods with circulation of Alpha or Delta [1, 7, 9, 10, 21]. The shorter time elapsed between the first and the second dose in the study sample for the Delta period is unlikely to explain this lower estimated effectiveness, since vaccine effectiveness did not vary by time since vaccination in the Omicron period.
In this analysis we estimated a higher effectiveness (95% CI) of a second dose among individuals with a prior SARS-CoV-2 infection (62.2%; 58.2–66.4%) than the effectiveness of a third dose in individuals without previously documented infection (51%; 50–52%) in the same population [22]. Also, the absolute risk of infection between days 7–34 of follow-up was lower in people with previous infection and a single vaccine dose (24.0 per 1000) than in people without previously documented infection with 2 vaccine doses (36.2 per 1000). The effectiveness was higher for an additional dose of the mRNA-1273 vaccine than of the BNT162b2 vaccine, which is consistent with previous findings in studies of individuals without documented infection [22, 23]. Effectiveness was estimated to be greater for heterologous regimens, especially when the first dose was BNT162b2, than for homologous regimens.
Like previous studies [1, 24], our findings indicate that the protection afforded by 1 vaccine dose in people with previous infection does not wane after a few months. For example, we found a risk of infection of 24.0 per 1000 in people who had received their vaccine dose 91–150 days ago and of 24.6 per 1000 in those who had received it more than 180 days ago. Also, the estimated effectiveness of the second vaccine dose did not vary depending on the elapsed interval since the first dose, as was also seen in a recent study in the United Kingdom [24].
Our study has some limitations. First, the higher number of tests in the first days of follow-up in the control group suggests that some symptomatic individuals who were already infected could not be excluded from that group. However, this bias is transient and of limited magnitude, and thus unlikely to influence our estimates of effectiveness. Second, self-tests are not consistently captured in the registry. Although the similar number of recorded tests during follow-up in the 2 groups makes it unlikely that this misclassification is differential, the absolute risks may be underestimated. Third, we could only estimate the effectiveness through 34 days of follow-up. The duration of protection against Omicron conferred by an additional vaccine in those people with previous infection will need to be monitored over time. Finally, severity of disease and/or symptoms were not assessed.
In conclusion, our study suggests that a second dose increases protection against infection with the SARS-CoV-2 Omicron variant up to 34 days after administration among individuals previously infected with another variant and who had received 1 vaccine dose.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Susana Monge, Department of Communicable Diseases, National Centre of Epidemiology, Institute of Health Carlos III, Madrid, Spain; CIBER on Infectious Diseases, Madrid, Spain.
Ayelén Rojas-Benedicto, Department of Communicable Diseases, National Centre of Epidemiology, Institute of Health Carlos III, Madrid, Spain; CIBER on Epidemiology and Public Health, Madrid, Spain; National Distance Education University, Madrid, Spain.
Carmen Olmedo, Vaccines Division, General Directorate of Public Health, Ministry of Health, Madrid, Spain.
Elisa Martín-Merino, Spanish Agency of Medicines and Medical Devices (AEMPS), Madrid, Spain.
Clara Mazagatos, Department of Communicable Diseases, National Centre of Epidemiology, Institute of Health Carlos III, Madrid, Spain; CIBER on Epidemiology and Public Health, Madrid, Spain.
Aurora Limia, Vaccines Division, General Directorate of Public Health, Ministry of Health, Madrid, Spain.
María José Sierra, CIBER on Infectious Diseases, Madrid, Spain; Centre for the Coordination of Heath Alerts and Emergencies, General Directorate of Public Health, Ministry of Health, Madrid, Spain.
Amparo Larrauri, Department of Communicable Diseases, National Centre of Epidemiology, Institute of Health Carlos III, Madrid, Spain; CIBER on Epidemiology and Public Health, Madrid, Spain.
Miguel A Hernán, CAUSALab and Departments of Epidemiology and Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
IBERCovid, Department of Communicable Diseases, National Centre of Epidemiology, Institute of Health Carlos III, Madrid, Spain.
IBERCovid:
David Moreno, Manuel Méndez Díaz, Ismael Huerta González, Antònia Galmés Truyols, Ana Barreno Estévez, Valvanuz García Velasco, Mª Jesús Rodríguez Recio, José Sacristán, Montserrat Martínez Marcos, Eliseo Pastor Villalba, María José Macías Ortiz, Ana García Vallejo, María Dolores Lasheras Carbajo, Aurelio Barricarte Gurea, Rosa Sancho Martínez, Eva María Ochoa, Mauricio Vázquez Cantero, Atanasio Gómez Anés, María Jesús Pareja Megía, Yolanda Castán, Manuel Roberto Fonseca Álvarez, Antonia Salvà Fiol, Hilda Sánchez Janáriz, Luz López Arce, María Ángeles Cisneros Martín, Frederic Jose Gibernau, Cesar Fernandez Buey, Katja Villatoro Bongiorno, Luis Lozano Mera, Fernando Santos Guerra, Jenaro Astray Mochales, Francisco Javier Francisco Verdu, Isabel García Romero, Rosa Oriza Bernal, Tomás Gómez Pérez, Salomé Hijano Villegas, Sergio Román Soto, Diana Gómez-Barroso, María Fé Lapeña, Virgilio Yagüe Galaup, Mercedes Alfaro Latorre, Marta Aguilera Guzmán, Belén Crespo Sánchez-Eznarriaga, Montserrat Neira León, and Noemí Cívicos Villa
Notes
Author contributions. S. M., M. A. H., and A. Larrauri conceived the idea for the study. S. M., A. R.-B., and M. A. H. designed the analysis, which was conducted by S. M. and A. R.-B. under the supervision of M. A. H. The collective author participated in data collection and critically reviewed study results. All authors participated in the interpretation of results and critically reviewed the content of the manuscript.
Members of IBERCovid (Investigación Basada en Registros de COVID-19). David Moreno, Manuel Méndez Díaz, Ismael Huerta González, Antònia Galmés Truyols, Ana Barreno Estévez, Valvanuz García Velasco, Mª Jesús Rodríguez Recio, José Sacristán, Montserrat Martínez Marcos, Eliseo Pastor Villalba, María José Macías Ortiz, Ana García Vallejo, María Dolores Lasheras Carbajo, Aurelio Barricarte Gurea, Rosa Sancho Martínez, Eva María Ochoa, Mauricio Vázquez Cantero, Atanasio Gómez Anés, María Jesús Pareja Megía, Yolanda Castán, Manuel Roberto Fonseca Álvarez, Antonia Salvà Fiol, Hilda Sánchez Janáriz, Luz López Arce, María Ángeles Cisneros Martín, Frederic Jose Gibernau, Cesar Fernandez Buey, Katja Villatoro Bongiorno, Luis Lozano Mera, Fernando Santos Guerra, Jenaro Astray Mochales, Francisco Javier Francisco Verdu, Isabel García Romero, Rosa Oriza Bernal, Tomás Gómez Pérez, Salomé Hijano Villegas, Sergio Román Soto, Diana Gómez-Barroso, María Fé Lapeña, Virgilio Yagüe Galaup, Mercedes Alfaro Latorre, Marta Aguilera Guzmán, Belén Crespo Sánchez-Eznarriaga, Montserrat Neira León, Noemí Cívicos Villa.
References
- 1. Hammerman A, Sergienko R, Friger M, et al. . Effectiveness of the BNT162b2 vaccine after recovery from Covid-19. N Engl J Med 2022; 386:1221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grupo de Trabajo Técnico de Vacunación COVID-19, de la Ponencia de Programa y Registro de Vacunaciones. Estrategia de vacunación frente a COVID 19 en España [Internet] . Available at:http://www.mscbs.es/profesionales/saludPublica/ccayes/alertasActual/nCov/vacunaCovid19.htm. Accessed 8 March 2022.
- 3. European Centre for Disease Prevention and Control (ECDC) . Technical report. Overview of the implementation of COVID-19 vaccination strategies and deployment plans in the EU/EEA. 31 January 2022 [Internet]. Available at:https://www.ecdc.europa.eu/sites/default/files/documents/Overview-of-COVID-19-vaccination-strategies-deployment-plans-in-the-EU-EEA-Jan-2022_1.pdf. Accessed 8 March 2022.
- 4. Gazit S, Shlezinger R, Perez G, et al. . The incidence of SARS-CoV-2 reinfection in persons with naturally acquired immunity with and without subsequent receipt of a single dose of BNT162b2 vaccine : a retrospective cohort study. Ann Intern Med 2022;175:674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahallawi WH, Fakher MH, Alsarani MA, Aljohani RH, Al-Mutabgani SA, Ibrahim NA. A single dose of SARS-CoV-2 vaccine primes a strong humoral immune response in COVID-19-recovered patients. Viral Immunol 2022;35:122–8. [DOI] [PubMed] [Google Scholar]
- 6. Wang Z, Muecksch F, Schaefer-Babajew D, et al. . Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021; 595:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mazzoni A, Di Lauria N, Maggi L, et al. . First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J Clin Invest 2021; 131:e149150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ali H, Alahmad B, Al-Shammari AA, et al. . Previous COVID-19 infection and antibody levels after vaccination. Front Public Health 2021; 9:778243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Gils MJ, van Willigen HDG, Wynberg E, et al. . A single mRNA vaccine dose in COVID-19 patients boosts neutralizing antibodies against SARS-CoV-2 and variants of concern. Cell Rep Med 2022; 3:100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shrestha NK, Burke PC, Nowacki ASet al. . Necessity of COVID-19 vaccination in persons who have already had COVID-19. Clin Infect Dis 2022; 75:e662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manisty C, Otter AD, Treibel TA, et al. . Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021; 397:1057–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saadat S, Rikhtegaran Tehrani Z, Logue J, et al. . Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA 2021; 325:1467–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lozano-Ojalvo D, Camara C, Lopez-Granados E, et al. . Differential effects of the second SARS-CoV-2 mRNA vaccine dose on T cell immunity in naive and COVID-19 recovered individuals. Cell Rep 2021; 36:109570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abu Jabal K, Ben-Amram H, Beiruti K, et al. . Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021; 26:2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Planas D, Saunders N, Maes P, et al. . Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022; 602:671–5. [DOI] [PubMed] [Google Scholar]
- 16. Ferguson NM, Ghani A, Cori A, Hogan A, Hinsley W, Volz E. Imperial College COVID-19 response team. Report 49: Growth, population distribution and immune escape from Omicron in England [Internet]. Available at:https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-49-omicron/. Accessed 7 June 2022.
- 17. Cheng SMS, Mok CKP, Leung YWY, et al. . Neutralizing antibodies against the SARS-CoV-2 Omicron variant following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med 2022; 28:486–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Collie S, Champion J, Moultrie H, Bekker L-G, Gray G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N Engl J Med 2022; 386:494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. European Centre for Disease Prevention and Control . Data on SARS-CoV-2 variants in the EU/EEA [Internet]. Available at:https://www.ecdc.europa.eu/en/publications-data/data-virus-variants-covid-19-eueea. Accessed 8 March 2022.
- 20. Kaplan EL, Meier Paul. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–81. [Google Scholar]
- 21. Lumley SF, Rodger G, Constantinides B, et al. . An observational cohort study on the incidence of SARS-CoV-2 infection and B.1.1.7 variant infection in healthcare workers by antibody and vaccination status. Clin Infect Dis 2022;74:1208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monge S, Rojas-Benedicto A, Olmedo C, et al. . The effectiveness of mRNA vaccine boosters for laboratory-confirmed COVID-19 during a period of predominance of the Omicron variant of SARS-CoV-2. Lancet Infect Dis 2022 Jun 2;S1473-3099(22)00292-4.
- 23. Andrews N, Stowe J, Kirsebom F, et al. . Effectiveness of COVID-19 booster vaccines against covid-19 related symptoms, hospitalisation and death in England. Nat Med 2022; 28:831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hall V, Foulkes S, Insalata F, et al. . Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med 2022;386:1207–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.