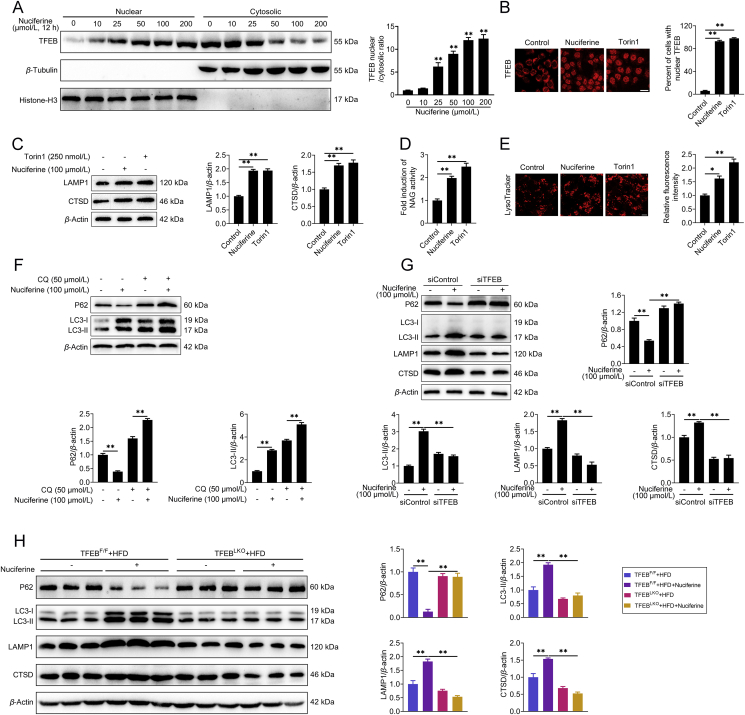
Figure 3.
Nuciferine activates ALP via TFEB. (A) Representative immunoblotting of nuclear and cytosolic TFEB in HepG2 cells and quantification of TFEB nuclear/cytosolic ratio and expressed as fold change relative to control group. HepG2 cells were treated with different concentrations of nuciferine (0, 10, 25, 50, 100 or 200 μmol/L) for 12 h. (B–E) HepG2 cells were treated with 100 μmol/L nuciferine for 12 h or 250 nmol/L Torin1 for 1 h. (B) HepG2 cells were analyzed by immunofluorescence and quantified to calculate the percentage of cells showing TFEB unclear localization. Scale bar, 20 μm. (C) Representative immunoblotting of LAMP1 and CTSD in HepG2 cells and quantification of LAMP1/β-actin, CTSD/β-actin and expressed as fold change relative to control group. (D) Relative lysosomal NAG activity. (E) Representative images of LysoTracker staining in HepG2 cells and quantification of fluorescence intensity and expressed as fold change relative to control group. Scale bar, 20 μm. (F) Representative immunoblotting of P62 and LC3 in HepG2 cells and quantification of P62/β-actin, LC3-II/β-actin and expressed as fold change relative to control (no treatment) group. HepG2 cells were pre-treated with 50 μmol/L chloroquine for 4 h and then treated with or without 100 μmol/L nuciferine for 12 h. (G) Representative immunoblotting of P62, LC3, CTSD and LAMP1 in HepG2 cells and quantification of P62/β-actin, LC3-II/β-actin, LAMP1/β-actin, CTSD/β-actin and expressed as fold change relative to control (transfection with siControl alone) group. HepG2 cells were transfected with siTFEB or siControl, then treated with or without 100 μmol/L nuciferine for 12 h. (H) Representative immunoblotting of P62, LC3, CTSD and LAMP1 in the livers from different groups and quantification of P62/β-actin, LC3-II/β-actin, LAMP1/β-actin, CTSD/β-actin and expressed as fold change relative to control (HFD-fed TFEBF/F mice) group. Mice were fed an HFD for 16 weeks. Nuciferine-treated mice were fed an HFD for 12 weeks and then fed an HFD containing 0.03% nuciferine for another 4 weeks. n = 6 mice per group were used to analyze the results. All experiments were repeated at least 3 times. Data were expressed as the mean ± SEM; ∗P < 0.05, ∗∗P < 0.01.