Fig. 2.
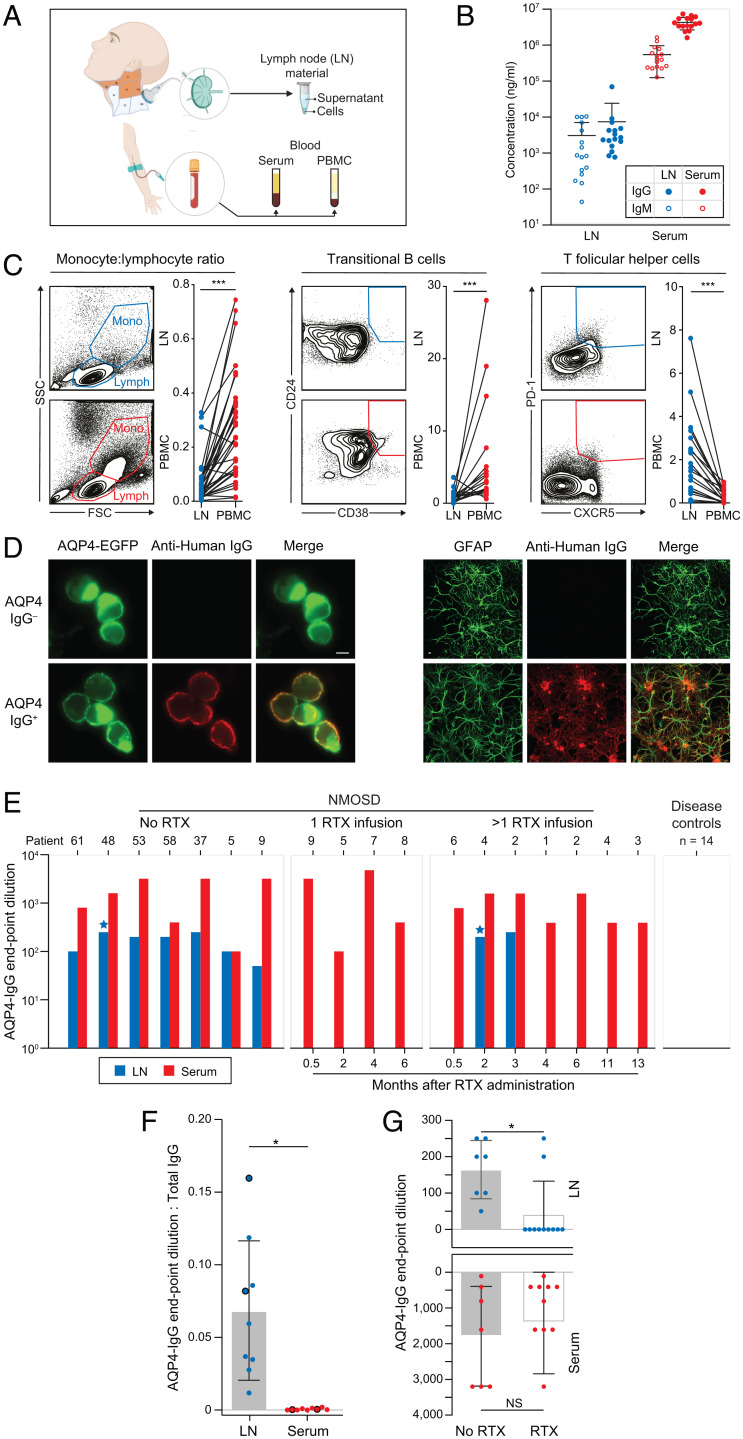
CLN aspirations contain AQP4 antibodies, which are abrogated after RTX administration. (A) CLNs across anatomical levels I, II, III, and V (orange) were aspirated under ultrasound guidance. Paired blood was obtained in parallel, resulting in cellular and soluble fractions from both sampled sites. (B) Markedly different levels of total IgG (filled symbols) and IgM (open symbols) were measured in LN aspirates (blue; IgG diluted at 1:800; IgM diluted at 1:100) versus matched sera of NMOSD patients (red; IgG diluted at 1:100,000; IgM diluted at 1:6,400). (C) Differences between PBMCs and LN cell populations are highlighted by the ratio of monocytes to lymphocytes (SSC = side scatter; FSC = forward scatter) and frequencies of both transitional B cells and Tfh cells (all P < 0.001, Wilcoxon signed-rank test), confirming that ultrasound LN sampling resulted in limited blood contamination of the LN aspirates. (D) AQP4-IgG was detected in LN aspirates by binding (anti-human IgG, red) to the surface of live AQP4–EGFP-transfected HEK293T cells (green; Right) and to the surface of live rat astrocytes (identified with GFAP, green; Left). (Scale bar, 10 µm). (E) AQP4-IgG levels in serum (red) and LN aspirates (blue) were measured in seven patients naive to RTX (of whom patients 5 and 9 went onto receive RTX), in four patients between 0.5 and 6 mo after one RTX infusion, and in seven patients between 0.5 and 13 mo after more than one RTX infusion (including two sampled longitudinally: patients 2 and 4). Blue stars indicate AQP4-IgM detected in two LN samples. (F) The ratios of AQP4-IgG levels (endpoint dilutions) to total IgG levels were compared between LN aspirates and sera (P = 0.02, Wilcoxon signed-rank test). Large dots highlight the two RTX-treated patients with detectable AQP4-IgGs from LN aspirates. (G) AQP4-IgG levels from LN aspirates (P = 0.04, Mann–Whitney U test) and serum (nonsignificant) in patients who did versus did not receive RTX; NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.