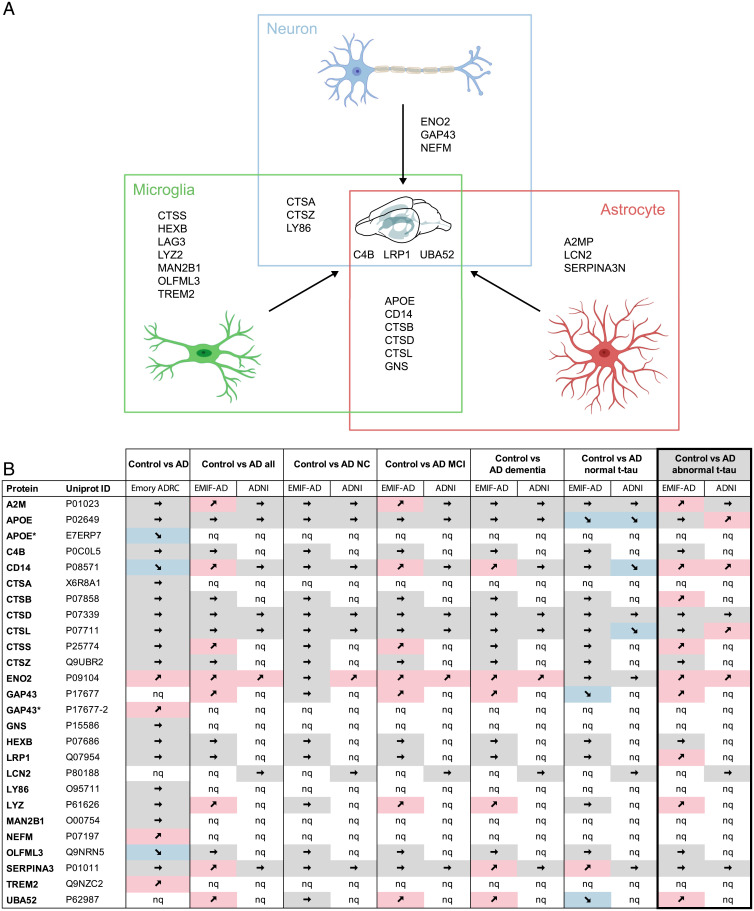
Fig. 7.
Common CSF protein changes in aged APPPS1 and A30P-αS mice are linked to glial cells and translate to human AD. (A) The majority of CSF proteins found to be increased in both mouse models (APPPS1 and A30P-αS) are linked to microglia (green) and/or astrocytes (red) and only a few are of neuronal origin (blue). See Results for details. Homologs of 24 of the 25 mouse proteins (all except LAG3) could also be measured in human CSF samples using MS-based technologies (compare with B). Partially created with bioRender. (B) Direct comparison of the murine CSF proteins with recently published human CSF proteome datasets including three different cohorts—Emory-ADRC (33) or EMIF-AD and ADNI (34)—revealed that several proteins of the mouse panel also show disease-related changes in AD patients. The best match between human and mouse data were seen for AD patients with abnormal CSF t-tau levels (based on study-specific cutoff values; bold frame). A total of 11 proteins showed a significant increase (9 in the EMIF-AD and additional 2 in the ADNI cohort) compared to healthy controls (P < 0.05; note that CD14 and ENO2 were increased in both cohorts). MCI, mild cognitive impairment; NC, normal cognition; nq, not quantified; ➚ increase, ➘ decrease, ➙ no significant change (*proteins highlighted with a star are [potential] isoforms of proteins listed in the table, according to UniProt). Note that due to stringent data filtering or targeted approach, only 707 and 204 proteins were relatively quantified in the EMIF-AD and ADNI cohort, respectively. Definition of AD in the EMIF-AD and ADNI cohort was based on abnormal CSF Aβ1–42 concentrations and cognitive function was assessed according to international consensus criteria (for details see ref. 34).