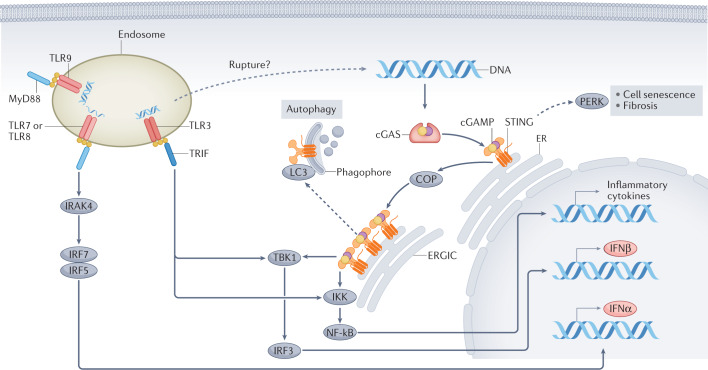
Fig. 2. Signalling pathways downstream of cGAS and of endosomal TLRs.
Binding to double-stranded DNA induces dimerization of cyclic GMP–AMP synthase (cGAS), which, in turn, leads to the synthesis of the second messenger 2′,3′-cyclic GMP–AMP (cGAMP). cGAMP binds with high affinity to stimulator of interferon genes (STING) leading to STING oligomerization and exit from the endoplasmic reticulum (ER); coatomer protein (COP) complexes facilitate the transport of STING. In the ER–Golgi intermediate compartment (ERGIC), STING recruits serine/threonine-protein kinase TBK1. The transcription factor interferon regulatory factor 3 (IRF3), which is a key TBK1 substrate, enters the nucleus following phosphorylation and promotes the transcription of IFNβ. Oligomerization of STING also enables activation of the inhibitor of nuclear factor-κB (NF-κB) kinase (IKK) complex, which promotes activation of the NF-κB transcription factor. NF-κB translocates to the nucleus where it promotes transcription of inflammatory cytokines tumour necrosis factor (TNF), IL-6 and IL-1β. By contrast, endosomal Toll-like receptors (TLRs) use the MyD88 or TRIF to generate platforms for downstream signal transduction. The DNA sensor TLR9 and the single-stranded RNA sensors, TLRs 7 and 8, activate IRF5 and IRF7 via Myd88 and IL-1 receptor-associated kinase 4 (IRAK4), which results in the synthesis of IFNα. The double-stranded RNA sensor TLR3, engages the TRIF platform, which leads to the activation of TBK1 and IKK, leading to IFNβ transcription. In non-classical pathway activation (dashed arrows), activated STING molecules on the ERGIC can bind to microtubule-associated protein 1 light chain 3 (MAP1LC3; also known as LC3) on the phagophore, which leads to STING degradation through autophagy. Moreover, STING activation in the ER might lead to PKR-like ER kinase (PERK) activation and subsequent cell senescence or fibrosis.