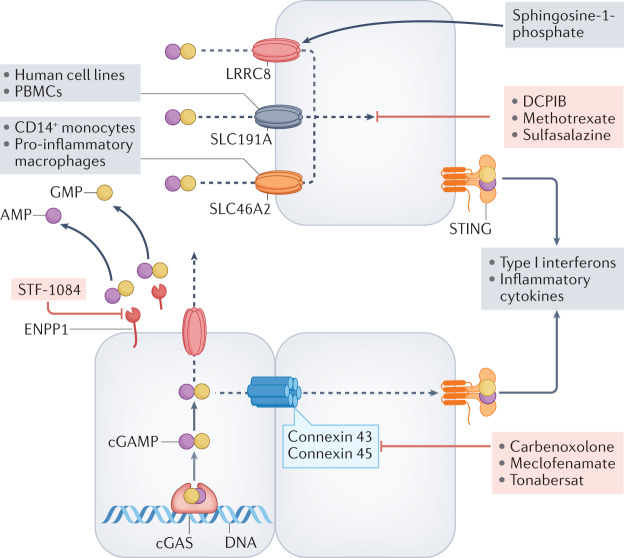
Fig. 4. Transport and metabolism of cGAMP.
Following activation, cyclic GMP–AMP synthase (cGAS) produces 2′,3′-cyclic GMP–AMP (cGAMP), which binds to stimulator of interferon genes (STING) within cells. The fate of intracellular cGAMP depends on its rate of synthesis, the cell type in which it is produced and the biological context. Excess cGAMP is transported out of cells and is hydrolysed by membrane-bound or soluble ectonucleotide pyrophosphatase phosphodiesterase 1 (ENPP1) to yield AMP and GMP in the extracellular space51,52. When production and export of cGAMP exceeds the catabolic capacity of ENPP1, cGAMP is imported into adjacent or distal cells through specific transporters, some of which can mediate bidirectional transport (LRRC8). The best-studied transporter in vascular endothelial cells is the volume-regulated anion (VRAC) channel LRRC8A:C heteromer, whereas the solute carrier SLC19A1 is functional in human cell lines and peripheral blood mononuclear cells, and SLC46A2 is active in CD14+ monocytes and pro-inflammatory macrophages60,62–64. In tumours, cGAMP can be transported between adjoining cells through gap junctions (for example, connexins 43 and 45)55,57,59, which can be blocked by the compounds shown. Several chemical compounds can block ENPP1 and cGAMP import as indicated. Sphingosine 1-phosphate can activate LRRC8 to potentiate cGAMP transport64.