Figure 1.
Clinical and epidemiological parameters of the DigiHero discovery cohort and patients with PASC
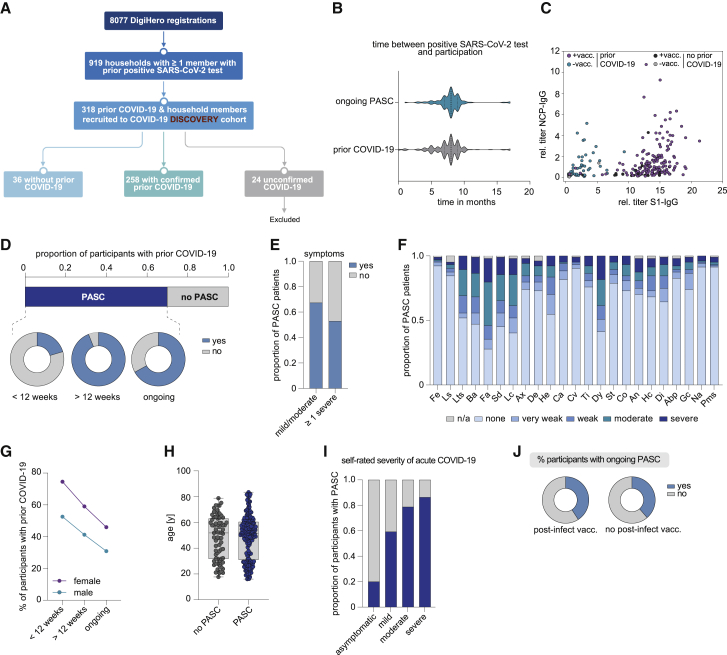
(A) Flow chart of the COVID-19 module of the DigiHero study.
(B) Median time from positive PCR or antigen test to participation in the module for the prior COVID-19 (n = 154) and ongoing PASC groups (n = 104).
(C) Plasma titer of antibodies directed against the S1 and NCP proteins of SARS-CoV-2 in individuals with or without SARS-CoV-2 vaccination (+vacc./−vacc.) and with or without prior COVID-19 from the DigiHero cohort.
(D) Proportion of DigiHero participants with self-reported PASC including duration of PASC symptoms after infection plus proportion of patients with ongoing symptoms at the time of blood sampling.
(E) Proportion of PASC patients with mild/moderate or at least one severe symptom.
(F) Severity of self-reported symptoms in PASC patients.
(G) Distribution of PASC duration between female and male study participants with prior COVID-19.
(H) Age distribution of DigiHero participants with or without PASC shown as box plot extending from the 25th to 75th percentiles. Median age is indicated as line. Bars represent range from smallest to highest value.
(I) Severity of acute COVID-19 in PASC patients. Abp, abdominal pain; An, angina; Ax, anxiety; Ba, body aches; Ca, coryza; Co, cough; Cv, conjunctivitis; De, depression; Di, dizziness; Dy, dyspnea; Fa, fatigue; Fe, fever; Gc, gastrointestinal complaints; He, headache; Hc, heart complaints; Lc, lack of concentration; Ls, lymph node swelling; Lts, loss of taste/smell; Na, nausea; Sai, self-reported severity of acute infection; SD, sleep disturbance; St, sore throat; Ti, tinnitus.
(J) Post-vaccination status of patients with ongoing PASC.