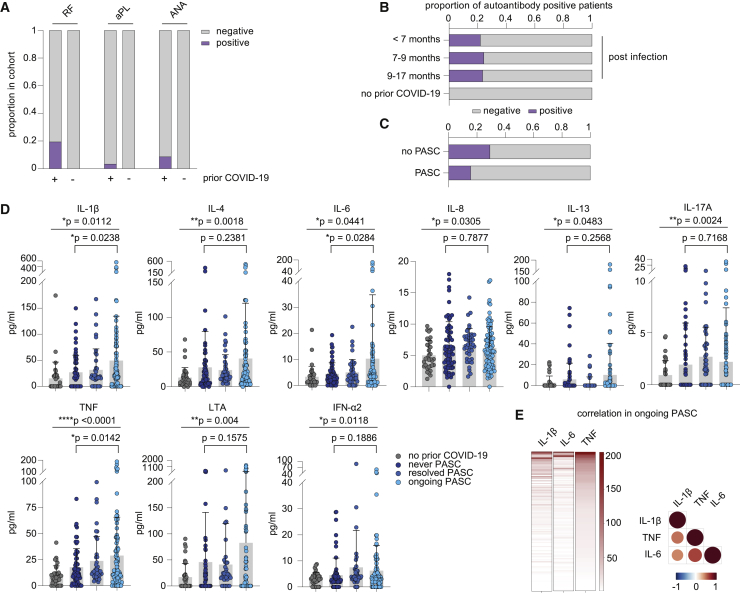
Figure 2.
Serological profiling of plasma from patients of the discovery cohort with ongoing PASC, after resolved SARS-CoV-2 infection and after resolved PASC
(A) Proportion of participants with rheumatic factor (RF), and antinuclear (ANA) and phospholipid autoantibodies (aPL) dependent on COVID-19 history. n (prior COVID-19) = 201; n (no prior COVID-19) = 36.
(B) Seroprevalence of autoantibodies over time in COVID-19 patients. n (<7 months) = 41; n (7–9 months) = 152; n (>9 months) = 28.
(C) Seroprevalence of autoantibodies in patients with ongoing PASC and individuals after infection without developing PASC. n (PASC) = 96; n (no PASC) = 65.
(D) Mean plasma cytokine levels of participants who never reported PASC post-infection (n = 65), with ongoing PASC (n = 96), with resolved PASC (n = 41), and participants without prior COVID-19 (n = 36). Error bars indicate ± SD. Statistical analysis: Welch’s ANOVA for comparison of all four groups and two-sided Welch corrected t test for comparison of never PASC versus ongoing PASC groups.
(E) Relation of IL-1β, IL-6, and TNF plasma levels in PASC patients displayed as heatmap (concentrations as pg/mL) and as correlation matrix.