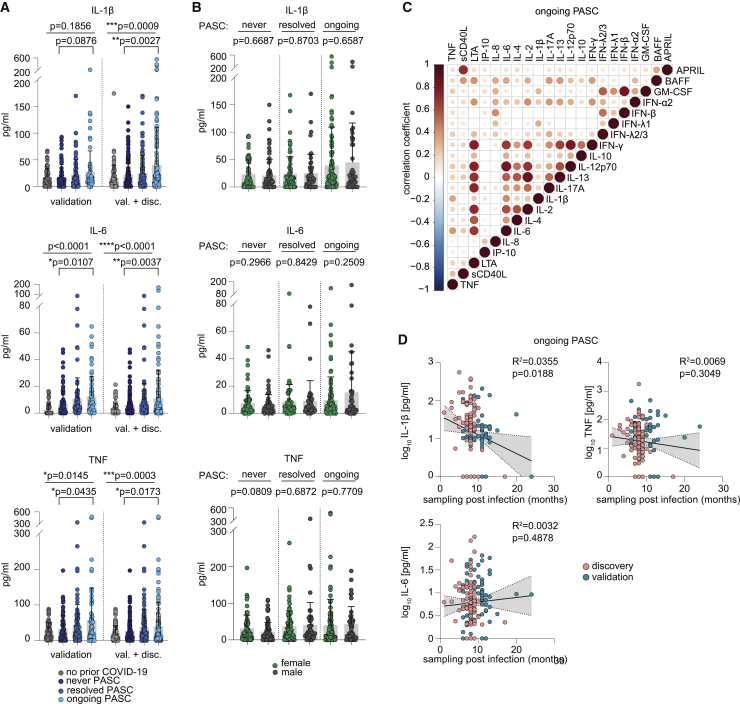
Figure 4.
Serological profiling of plasma from patients of the validation cohort with ongoing PASC, after resolved SARS-CoV-2 infection and after resolved PASC
(A) Mean plasma cytokine levels of participants with prior COVID-19 from the validation cohort who never reported PASC (n = 86), with ongoing PASC (n = 89), or with resolved PASC (n = 65) and participants without prior COVID-19 (n = 60), as well as plasma cytokine levels in the combined discovery and validation cohorts: n (never COVID-19) = 96; n (no PASC) = 150; n (resolved PASC) = 106; n (ongoing PASC) = 185. Error bars indicate ± SD. Statistical analysis: Welch’s ANOVA for comparison of all four groups and two-sided Welch corrected t test for comparison of never PASC versus ongoing PASC groups.
(B) Sex-dependent mean plasma cytokine levels in the never COVID-19 (71 females, 79 males), resolved PASC (77 females, 52 males), and ongoing PASC (116 females, 46 males) groups of the combined discovery and validation cohorts. Error bars indicate ± SD. Statistical analysis: two-sided Welch corrected t test.
(C) Correlation matrix of all cytokines in the combined ongoing PASC group (n = 185).
(D) Linear regression analysis of plasma cytokine levels and sampling time point post-infection in the combined ongoing PASC group (n = 185).