Table 1:
Library of novel triazole analogs synthesized from the one-pot synthesis, shown in Scheme 1 and their EC50 values
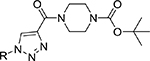
|
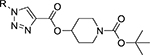
|
|||||
|---|---|---|---|---|---|---|
| R | Compound | Isolated yield (%) | EC50 (nM) | Compound | Isolated yield (%) | EC50 (nM) |
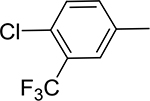
|
3a | 95 | 12.34±3.12 | 5a | 92 | 12.31 ±2.11 |

|
3b | 94 | 13.56±2.45 | 5b | 94 | 10.43±2.11 |

|
3c | 95 | 8.78±1.23 | 5c | 93 | 11.12±3.54 |
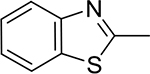
|
3d | 97 | 7.12±1.12 | 5d | 97 | 8.17±2.11 |

|
3e | 92 | 4.21±0.13 | 5e | 93 | 3.59±1.01 |

|
3f | 91 | 12.43±1.63 | 5f | 95 | 13.98±2.76 |
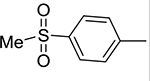
|
3g | 97 | 3.12±0.42 | 5g | 96 | 3.67± 1.01 |
