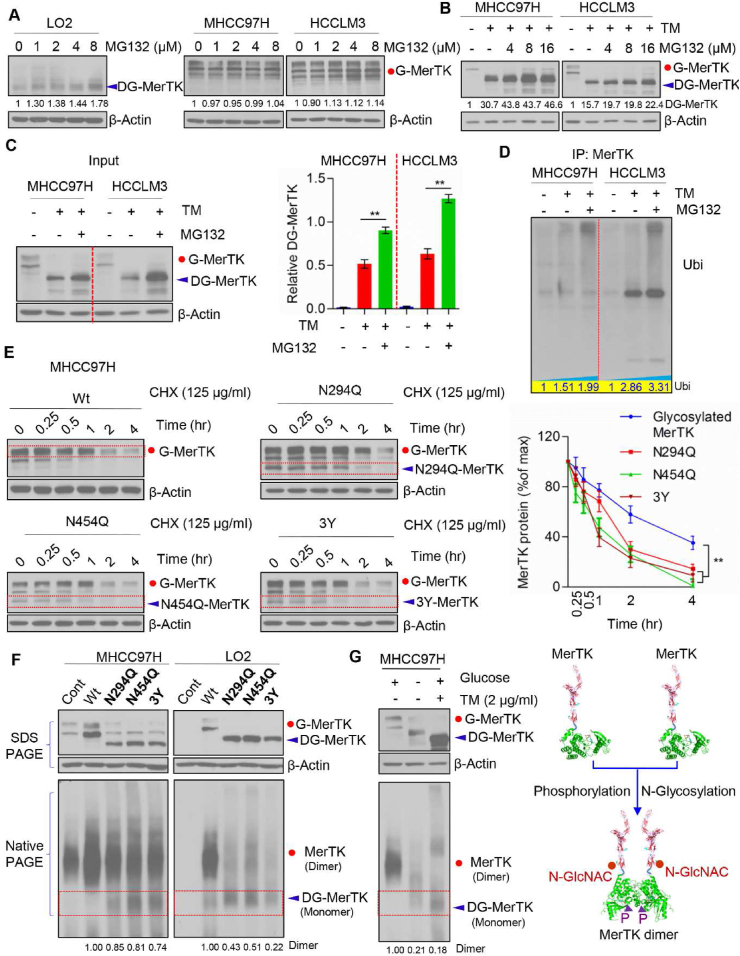
Fig. 7.
N-glycosylation modification stabilizes MerTK. (A) Western blot analysis of LO2, MHCC97H and HCCLM3 treated with indicated concentration of MG132 for 24 h followed by Western blot analysis with indicated antibodies. (B) MHCC97H and HCCLM3 cells were treated with TM (1 μg/ml) and/or indicated concentration of MG132 for 24 h followed by Western blot analysis with indicated antibodies. (C) Western blot analysis of MHCC97H and HCCLM3 cells treated with TM (1 μg/mL) and/or MG132 (125 μg/mL) (left panel) and relative DG-MerTK expression level was quantified by Image J and normalized to the β-Actin (right panel). Data are presented as mean ± SD of three independent experiments, statistical significance was assessed by Student's t-test, **P < 0.01. (D) MHCC97H and HCCLM3 cells with TM (1 μg/mL) and/or MG132 (125 μg/mL) treatment were subjected to MerTK immunoprecipitation (IP) followed by Western blot analyses with anti-ubiquitin. (E) WT and indicated MerTK mutants expressing in MHCC97H cells were treated with 125 μg/mL CHX at indicated intervals and detected by Western blot analysis. The intensity of MerTK protein was quantified using Image J and normalized to the β-Actin. Data are presented as mean ± SD of three independent experiments, statistical significance was assessed by One-way ANOVA with Bonferroni post-test, **P < 0.01. (F) SDS-PAGE and Blue Native PAGE of WT and indicated MerTK mutants expressing in MHCC97H and HCCLM3 cells to detect the MerTK dimerization. (G) SDS-PAGE and Blue Native PAGE of endogenous MerTK in MHCC97H cells treated with or without TM or in presence or absence of glucose (left panel). Proposed model of N-glycosylation and phosphorylation modification modulating MerTK homodimerization (right panel).