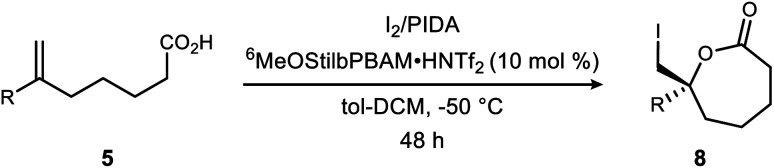
Extension of the BAM-catalyzed iodolactonization to ε-membered lactonesa.
| ||||
|---|---|---|---|---|
| Entry | 5/8 | R | eeb (%) | Yieldc (%, isolated) |
| 1 | a | C6H5 | 88 | 71 |
| 2 | b | 2Np | 87 | 64 |
| 3 | c | p MeC6H4 | 77 | 87 |
| 4d | d | m MeC6H4 | 84 | 76 |
| 5 | e | o MeC6H4 | — | Trace |
| 6 | f | p FC6H4 | 93 | 65 |
| 7 | g | m FC6H4 | 93 | 53 |
| 8e | h | m ClC6H4 | 96 | 21 |
| 9 | i | p ClC6H4 | 91 | 70 |
| 10 | j | p CF3C6H4 | 81 | 48 |
| 11f | k | 3,5(CF3)2C6H3 | 10 | 10 |
| 12 | l | m CHOC6H4 | 94 | 54 |
| 13 | m | p PhC6H4 | 84 | 72 |
| 14 | n | m MeOC6H4 | 90 | 63 |
| 15 | o | p MeOC6H4 | 49 | 53 |
| 16 | p | p (tBu)C6H4 | 83 | 66 |
| 17 | q | 5-(2-Methoxypyridine) | 75 | 73 |
| 18 | r | Me | 63 | 42 |
| 19 | s | Et | 72 | 58 |
| 20 | t | H | 44 | 24g |
| Substrate | Product | |||
| 21 |

|
10 | 87 | 70 |
| 22 |

|
12 | 65 | 90 |
| 23d | 92 | 19 | ||
| 24h | h | m ClC6H4 | 96 | 26 |
| 25h | n | m MeOC6H4 | 87 | 61 |
General experimental details: the oxidant (100 mol%), iodine (100 mol%), and carboxylic acid were combined in solvent and stirred for 48 hours prior to workup and analysis; see ESI for complete details.
Measured by HPLC using a chiral stationary phase.
Isolated yield after chromatography. Although column chromatography was successfully used at room temperature, many of the lactone products were noted to turn yellow, red, and then brown during final concentration in pure form. As a result, care should be taken to avoid heating during workup, and storage of pure materials at low temperature (<0 °C), or as a frozen solution in benzene, is highly recommended as standard practice. Yields measured from the crude reaction mixture (internal standard, 1H NMR) were routinely higher.
Reaction temperature = −20 °C.
Reaction time = 4 days.
Reaction temperature = 25 °C.
Measured by 1H NMR analysis of the crude reaction mixture due to product instability.
Results at 1 mmol scale for substrate.
