Fig 2.
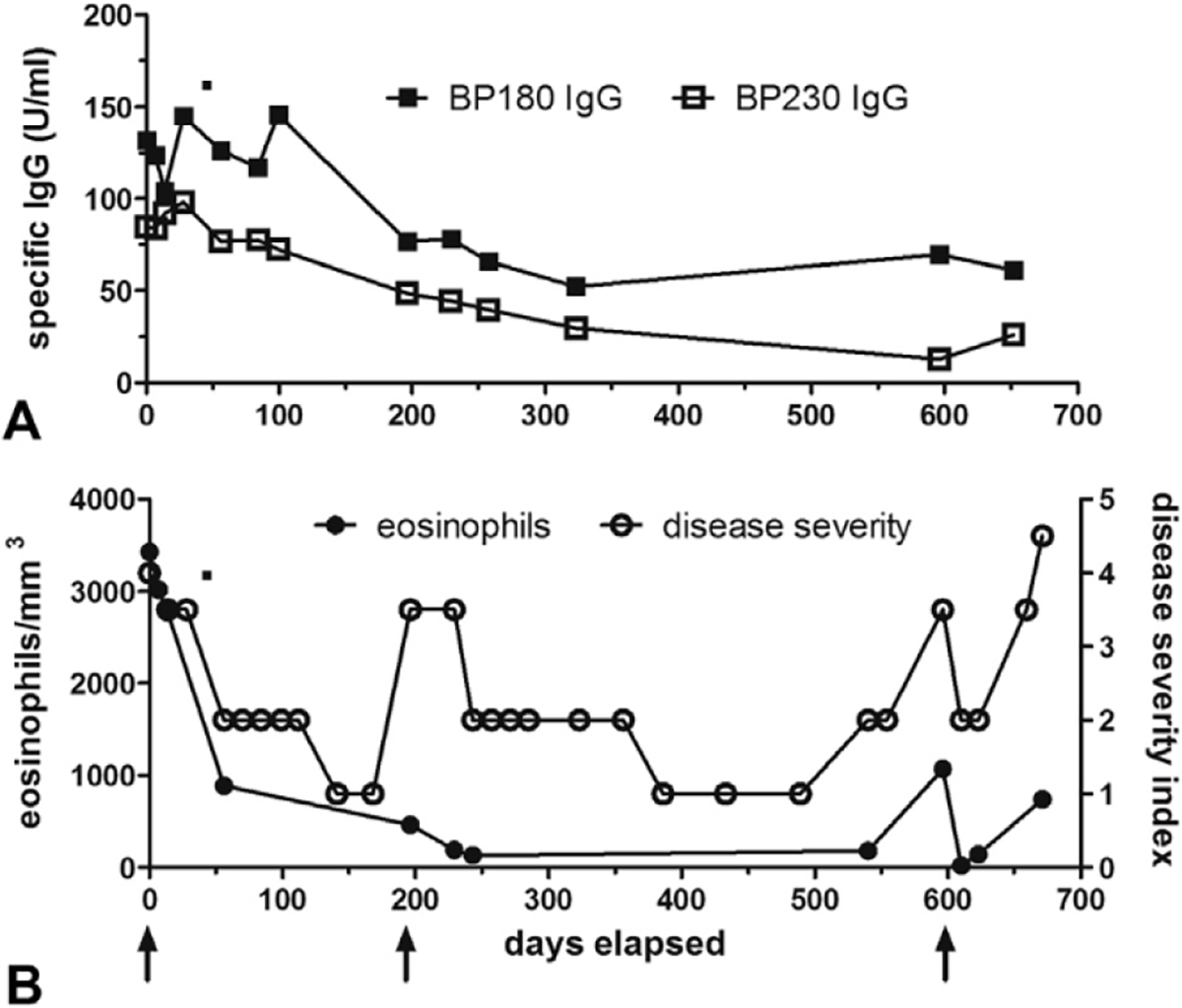
Patient 4: autoantibody levels, eosinophil counts, and disease severity throughout omalizumab therapy. Patient 4 was assessed during 3 separate courses of omalizumab; arrows indicate the first dose of omalizumab in her treatment cycles. Bullous pemphigoid (BP)180 and BP230 IgG (A) autoantibody levels were measured in serum samples by enzyme-linked immunosorbent assay (MBL International, Woburn, MA). Disease activity was assessed on a 4-point scale: 4.5/4.0 = generalized disease >10% total body square area with/without immunosuppressive medications; 3–5/3 0 = localized disease <10% total body square area with/without medications; 2 = remission with medications; 1 = remission without medications. Peripheral eosinophil counts (B) paralleled disease activity more closely than autoantibody levels.
