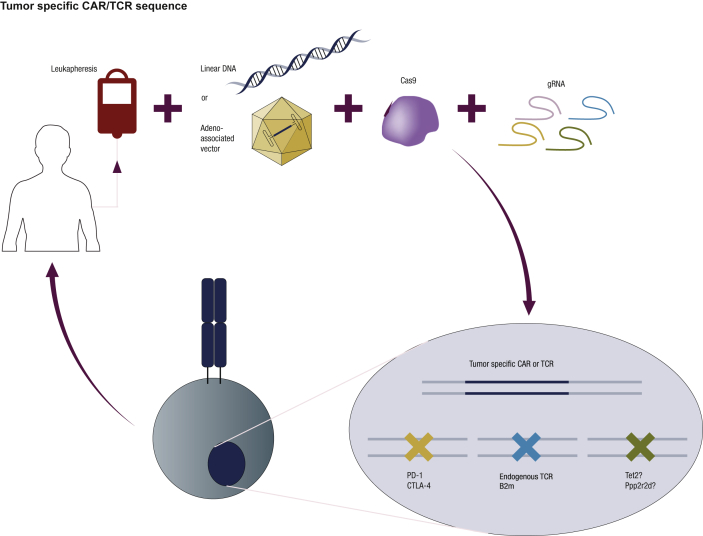
Figure 1.
T-cell gene editing with multiplex knock-out and endogenous T-cell receptor (TCR) replacement. Peripheral blood mononuclear cells, or specific T-cell subsets, can be electroporated to deliver Cas9 protein and one or multiple gRNAs complexed as ribonucleoproteins (RNPs). To replace endogenous TCRs with tumor-specific TCRs or chimeric antigen receptors (CARs), double- or single-stranded DNA can be used as a homology-directed repair (HDR) template and delivered together with the RNPs. Alternatively, adeno-associated vectors can also be used as HDR templates by infecting the modified cells after electroporation. Gene editing combining knock-in and knock-out with multiple gRNAs will allow generation of off-the-shelf allogeneic tumor-targeted T cells (endogenous TCR-β2M-) or T cells with enhanced antitumor potency (PD-1-, CTLA-4-). Other candidate genes, such as TET2 and PPP2R2D, are being knocked-out to investigate whether their absence will increase the potency of T cells.