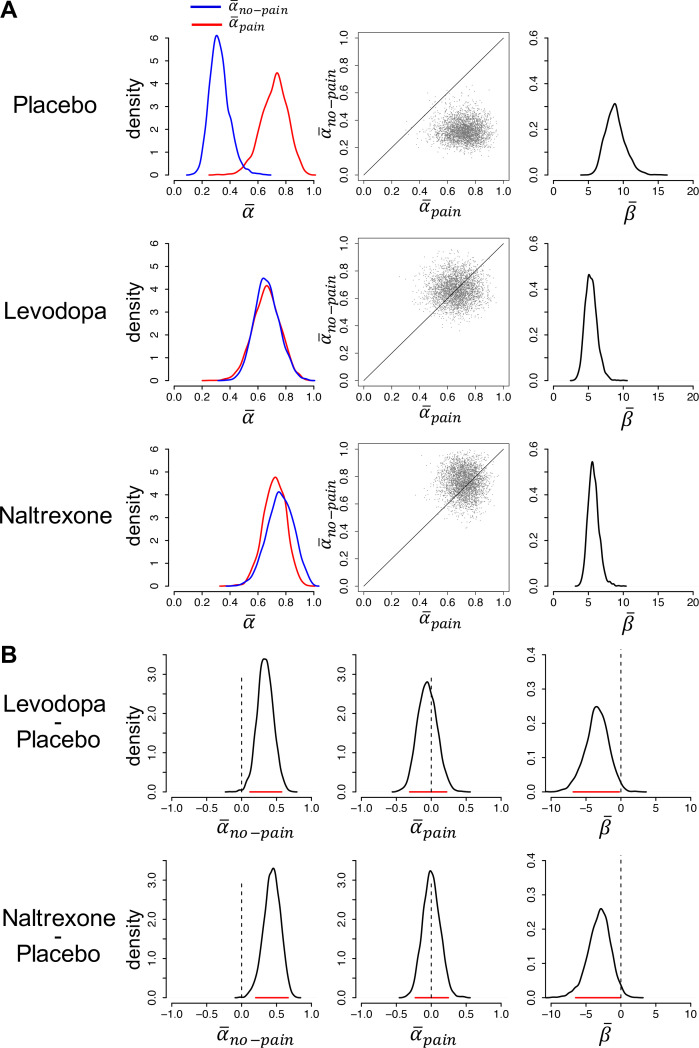
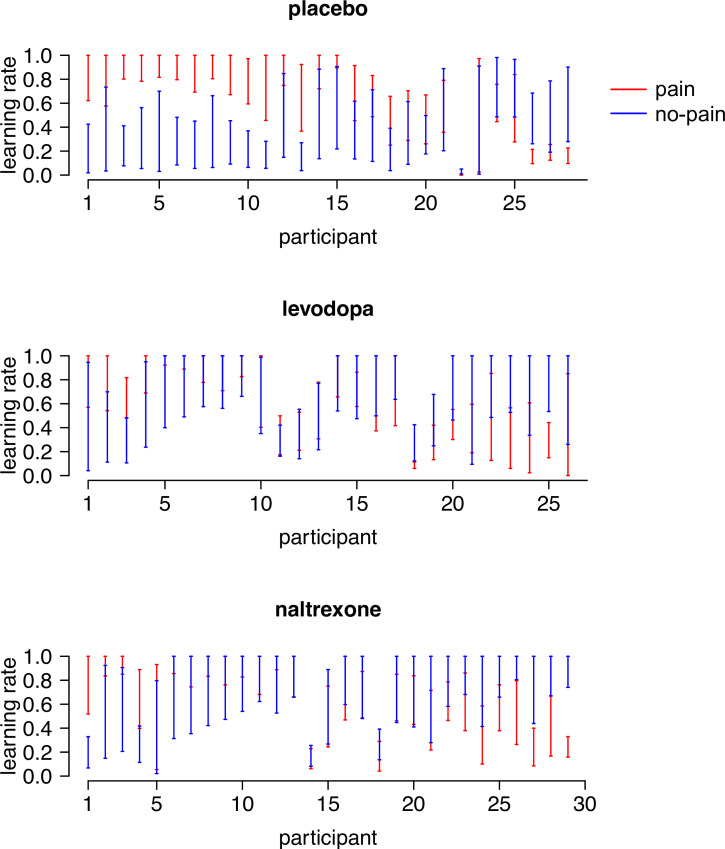
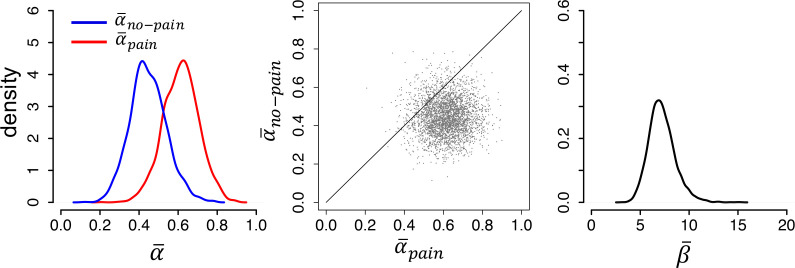
Figure 2. Model parameters.
(A) Posterior distributions of the parameters’ group-level means for each group (left and right panels). Parameters and are learning rates for avoided and received pain outcomes, respectively; parameter is the inverse-temperature parameter. The middle panels are joint density plots of and (dots are samples from the Markov chain Monte Carlo [MCMC]), showing that is reliably greater than in the placebo group only. (B) The difference between the posterior distributions for each drug group vs. the placebo group, showing that is greater and is smaller in both drug groups compared to the placebo group. Red lines indicate 95% highest density intervals (HDIs).