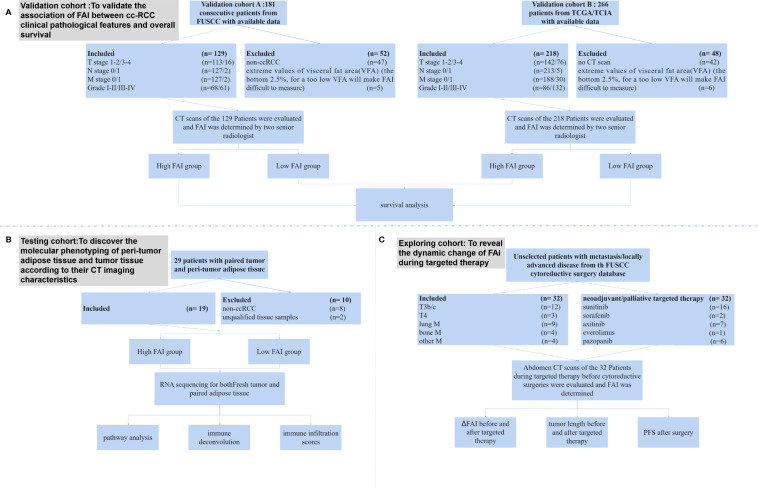
Figure 1.
The study design, inclusion criteria, and participants. (A) The validation cohorts were composed by patients from the FUSCC database (FAI cohort A) and TCGA/TCIA database (FAI cohort B). We excluded patients who did not have CT scans and those who had extreme values of VFA. The final included patients were 129 from the FUSCC cohort and 218 from the TCGA/TCIA cohort. (B) The imaging genomics cohort prospectively included consecutive unselected patients from FUSCC who had nephrectomy or nephron sparing surgery. After excluding 8 patients who were not clear cell RCC and 2 patients with unqualified tissue samples, the final cohort included 19 patients. (C) The treatment response cohort included patients from a FUSCC cytoreductive surgery database. All patients had CT scans of the kidney tumor before and after two months of neoadjuvant/palliative targeted therapy, and then they undergone nephrectomies.