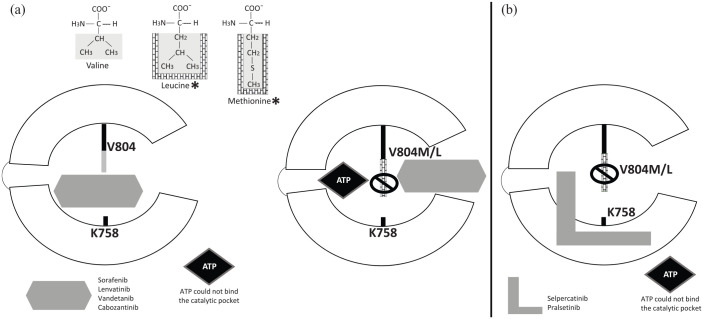
Figure 3.
Mechanism of resistance to RET inhibition: Target modification V804M/L gatekeeper mutation. RET tyrosine kinase domain catalytic cleft as shown in Figure 2. (a) Asterisks indicate structural differences of leucine and methionine with bulkier side chains compared with nonmutant valine residue. The bulkier side chains (in brick wall) prevent multitargeted tyrosine kinase inhibitors (sorafenib, lenvatinib, vandetanib, cabozantinib) to communicate between the front and back clefts of the RET receptor kinase domain catalytic cleft. (b) Selpercatinib and pralsetinib can overcome gatekeeper mutations as they bind to the front and back clefts of the RET kinase domain catalytic pocket without going through the gate between V804 and K758. Instead, they pass around the gate wall K758 residue to access the back pocket.