Figure 3.
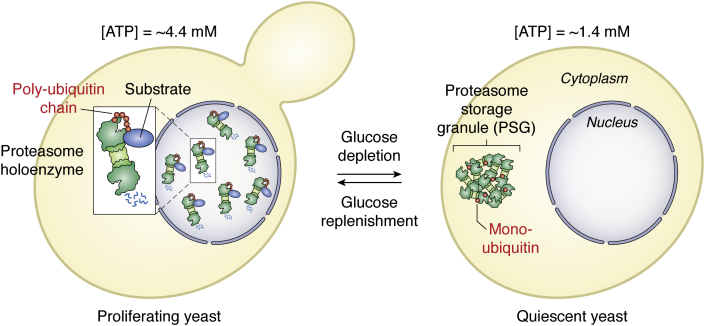
Proteasome movement between proliferation and quiescence in yeast. In proliferating cells with high ATP, proteasome holoenzymes are mainly localized in the nucleus and engaged in the degradation of polyubiquitinated proteins. With glucose depletion toward quiescence, the ATP level declines. Proteasomes then exit the nucleus through nuclear pores and are sequestered into PSGs, which move as membraneless organelles through the cytoplasm (21). PSGs are mainly composed of proteasomal components and monoubiquitin (90). Since proteasome holoenzymes are unstable at low ATP, CP and RP are dissociated in lysates of quiescent yeast cells (26). With the addition of glucose, cells receive the signal to resume growth. The ATP level increases and PSGs clear within a few min. Proteasomes are rapidly relocated to the nucleus where ATP- and ubiquitin-dependent proteolysis promotes cell cycle progression. CP, core particle; PSG, proteasome storage granule; RP, regulatory particle.