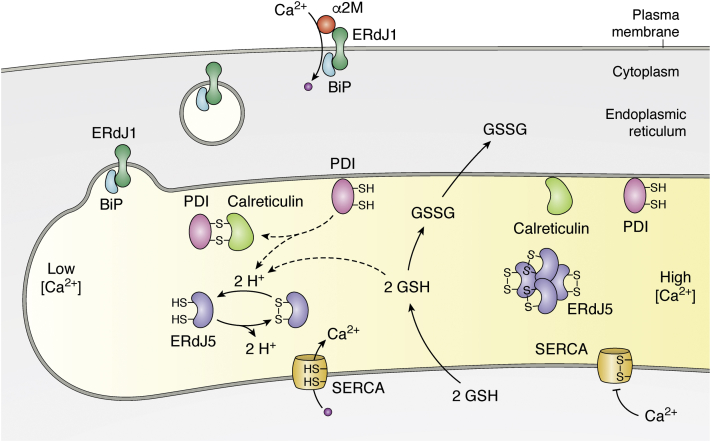
Figure 2.
Schematic illustration of the function of ERdJ1 and ERdJ5 in controlling Ca2+influx. ERdJ1 targets the chaperone BiP to the plasma membrane where it functions as a receptor for α2-macroglobulin (α2M) to enable Ca2+ influx across the plasma membrane into the cytosol. Within the ER, the cochaperone ERdJ5 regulates Ca2+ influx by reducing disulfide bonds of SERCA thereby activating SERCA, which pumps Ca2+ into the ER. The color shading of the ER lumen symbolizes different Ca2+ concentrations in the ER with low [Ca2+] at the left and high [Ca2+] at the right. Under normal Ca2+ concentrations, PDI1A prevails in its reduced and ERdJ5 in its oxidized form, in which it forms large protein complexes. The reductive milieu, required to reduce ERdJ5, is presumed to be provided by PDI, bound to calreticulin and by GSH, which is transported into the ER when Ca2+ concentrations in the ER decrease. Reduced ERdJ5 increases the reductive power to reduce and activate the ATPase activity of the Ca2+ channel SERCA, which—in its reduced state—can pump Ca2+ into the ER. ER, endoplasmic reticulum; PDI, protein disulfide isomerase; SERCA, sarcoplasmic/endoplasmic reticulum calcium ATPase.