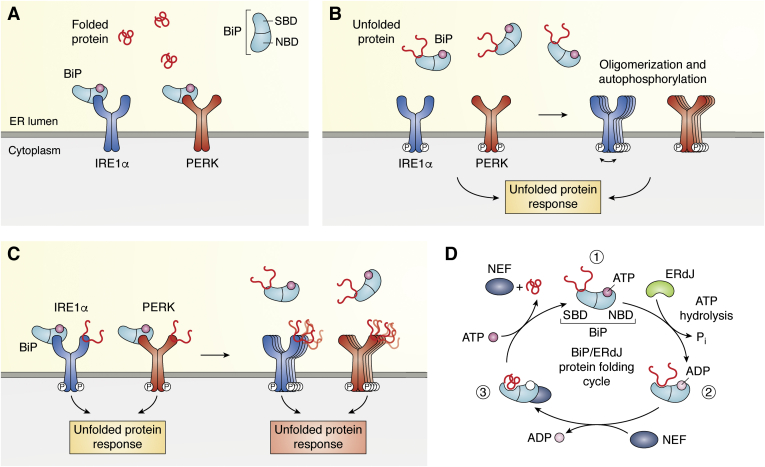
Figure 3.
Induction of ER-stress by misfolded proteins.A, BiP has a substrate-binding domain (SBD) and a nucleotide-binding domain (NBD). IRE and PERK are transmembrane proteins with high homologous luminal domains, which are silenced by BiP-NBD binding under normal conditions, when nascent proteins are folded properly (red coiled lines). B, in ER-stressed cells, misfolded proteins accumulate and engage luminal BiP for refolding. By binding of the misfolded proteins to the SBD, BiP dissociates from the luminal domains, enabling the oligomerization and autophosphorylation of the transmembrane proteins thereby activating the UPR. C, according to the extended model, misfolded proteins bind directly to the luminal domains of the transmembrane proteins, enabling their activation and stimulation of the UPR. Under more severe stress conditions, even BiP, which is usually bound to the transmembrane domains, binds unfolded protein and dissociates from the respective luminal domains thereby enforcing the UPR. D, misfolded proteins, bound by BiP, are subjected to a cycle to enable their refolding. This refolding, ATP-consuming chaperoning is assisted by ERdJ proteins and nucleotide exchange factors (NEF). In a first step (1), BiP is shown in its ATP-bound state, in which binding affinity to the substrate (unfolded, native protein or misfolded protein) is low. In a second step (2), ERdJ proteins, as cochaperones, bind to BiP and stimulate ATP hydrolysis. In the ADP-bound state, the affinity of BiP to the substrate is increased. When protein folding has been completed, ADP is exchanged for ATP by the nucleotide exchange factor (NEF) resulting in release of the substrate protein (3). ER, endoplasmic reticulum; UPR, unfolded protein response.