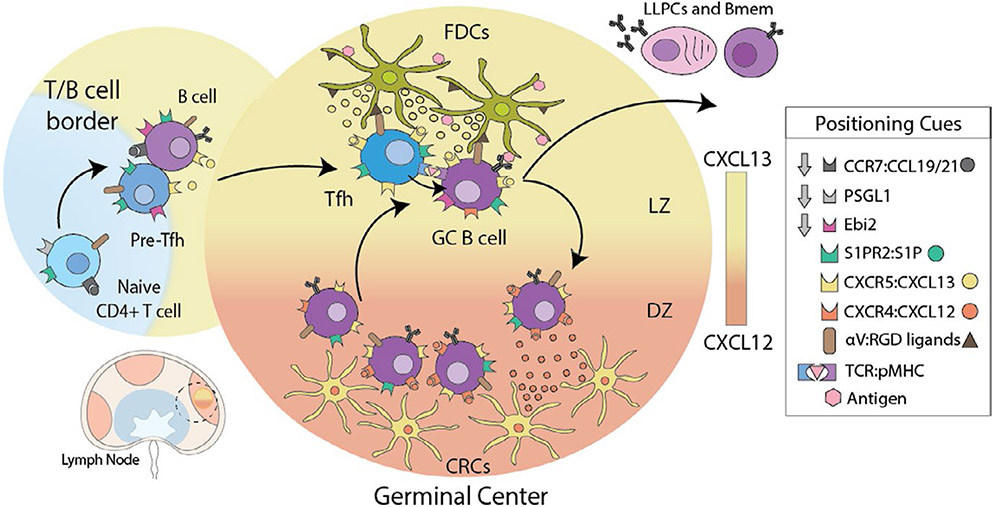
FIGURE 3.
Germinal Center guidance signals. Dendritic cell-activated CD4 T cells downregulate receptors for T cell zone localization cues (PSGL-1 and CCR7), while increasing the expression of receptors that bind follicular cues (Ebi2 and CXCR5) enabling pre-Tfh to re-locate to the T-B border and interfollicular zone to interact with B cells. Stable interactions with B cells presenting cognate antigen result in the reinforcement of the Tfh program and migration into GCs, directed by expression of S1PR2 and repression of EBI2 chemotactic receptors. GC localization of Tfh is further refined by physical interactions with B cells, controlled by surface receptors that can either have an attractant or retention function. Expression of integrin αVβ3 enhances Tfh localization to GC through interactions with FDC coated with RGD ligands. After B cells receive T cell help in the GC they upregulate CXCR4 and move toward a gradient of CXCL12 expressed by dark zone (DZ) CXCL12-expressing reticular cells (CRC). Within the DZ, B cells proliferate before downregulating CXCR4 and becoming responsive to CXCL13. They return to the light zone (LZ) by following a follicular dendritic cell (FDC)-generated CXCL13 gradient for further rounds of T cell help and somatic hypermutation. B cells that survive this rigorous process of mutation and selection in the GC differentiate into either long-lived plasma cells (LLPCs) or memory B cells (Bmem)