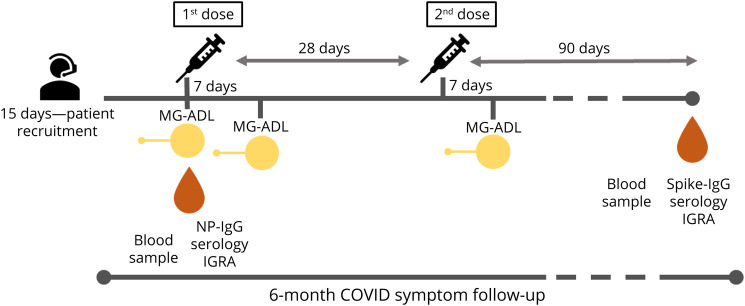
Figure 1. Study Concept.
Patients were prospectively recruited by telephone call over a period of 15 consecutive week days. The baseline MG-ADL score and a blood sample to assess NP-IgG serology and IGRA was obtained before the first dose was administered. Seven days after the first dose, a second MG-ADL score was obtained. The second dose of the vaccine was administered 28 days after the first dose, following the manufacturer's recommendations. A third MG-ADL score was administered a week after the second dose. For all 3 MG-ADL scores, patients were asked about symptoms in the previous 7 days to score each MG-ADL item. A second blood sample was obtained 90 days after the second dose, and IGRA and spike-IgG were determined. During a 6-month-follow-up, COVID-19 symptoms were telematically assessed in all patients. COVID-19 = coronavirus disease 2019; IgG = immunoglobulin G; IGRA = interferon gamma release assay; MG-ADL = myasthenia gravis activities of daily living score; NP-IgG serology = SARS-CoV-2 nucleocapsid protein antibodies serology; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; spike-IgG serology = SARS-CoV-2 spike protein 1 IgG antibodies serology.