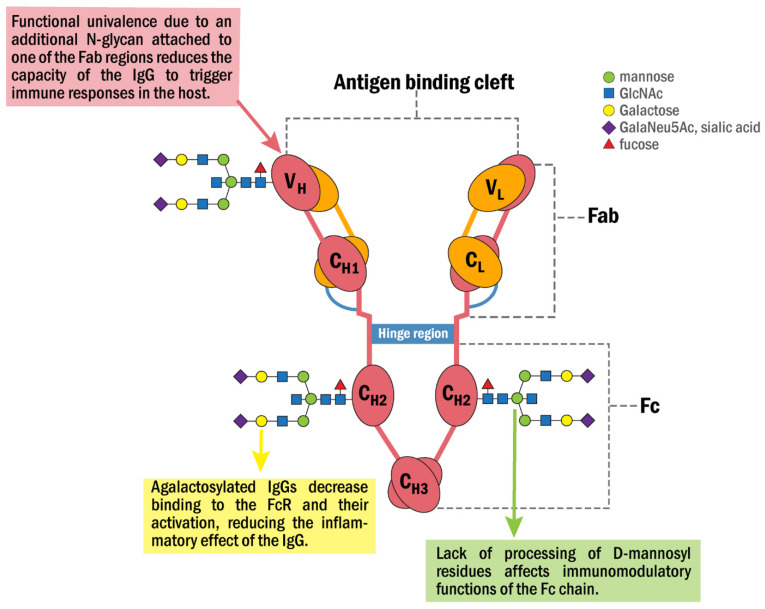
Figure 1.
Schematic representation of the anti-GRP78 IgG molecular structure and its associated N-glycan structures identified in cancer patients. It consists of two interconnected heavy (H) and two light chains (L) with two domains that infer different properties, the fragment antigen-binding (Fab) and fragment crystallizable (Fc) domains. The Fab domain is responsible for recognizing and binding the antigen. The Fc domain contains two glycans attached to Asn297 in conserved regions of the CH2 domain. The Fc domain binds to the FcR on natural killers and other inflammatory cells. In cancer, the Fab region is asymmetrically glycosylated with one additional N-glycan that converts the anti-GRP78 IgG into a univalent molecule that binds the antigen with a decreased affinity, preventing it from triggering some IgG-linked functions of the immune response such as complement fixation, phagocytic activity and antigen clearance. Moreover, the N-glycans of the Fc region are aberrantly glycosylated, mainly at the levels of D-mannosylation and galactosylation with a reduction in the affinity of the antibody for the FcR and its activation, thus inhibiting the inflammatory effects of the IgG.