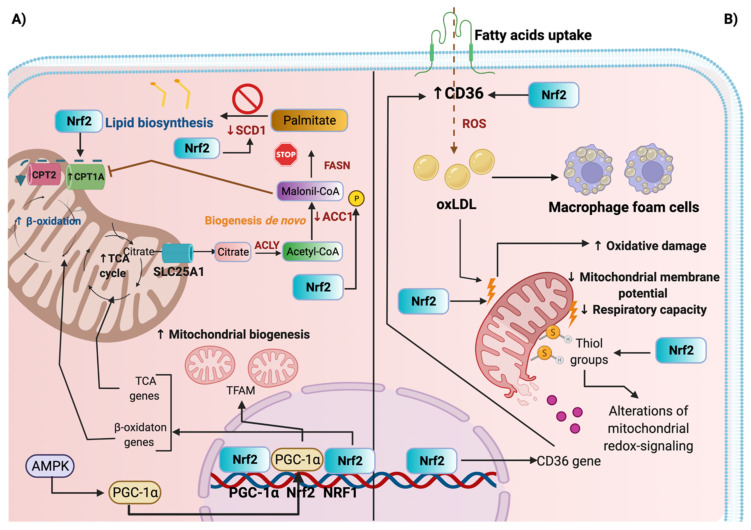
Figure 4.
Nuclear factor erythroid 2-related factor 2 (Nrf2) activation and mitochondria. (A) Nrf2 upregulates fatty-acid catabolism via β-oxidation, a process occurring in the mitochondria by inducing the expression of carnitine palmitoyltransferase 1 (CPT1), the rate-limiting enzyme in this mechanism. In contrast, Nrf2 inhibits acetyl-CoA carboxylase 1 (ACC1) via phosphorylation, preventing lipid biosynthesis. Furthermore, Nrf2 inhibits stearoyl-CoA desaturase. Nrf2 also induces the expression of the enzymes involved in the tricarboxylic-acid (TCA) cycle, upregulating this pathway. Nrf2 participates in mitochondria biogenesis by interacting with the peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α) and nuclear respiratory factor 1 (NRF1), promoting the expression of transcription factor A, mitochondrial (TFAM). Moreover, NRF1 contains at least four binding sites for Nrf2, participating in antioxidant response. (B) Nrf2 overactivation might induce the overexpression of cluster of differentiation 36 (CD36), the principal receptor to fatty-acid uptake, leading to the accumulation of low-density lipoproteins (LDLs) in the cytosol. LDLs are lipids with a major propensity for oxidation, forming oxidized LDLs (oxLDLs), further damaging the mitochondria. Moreover, macrophages engulf oxLDLs, which causes macrophage foam-cell formation. Nrf2 upregulation also induces mitochondrial membrane potential, decreases respiratory capacity, and alters mitochondrial thiol protein groups, leading to the alteration of mitochondrial redox signaling pathways. The figure was created using BioRender.