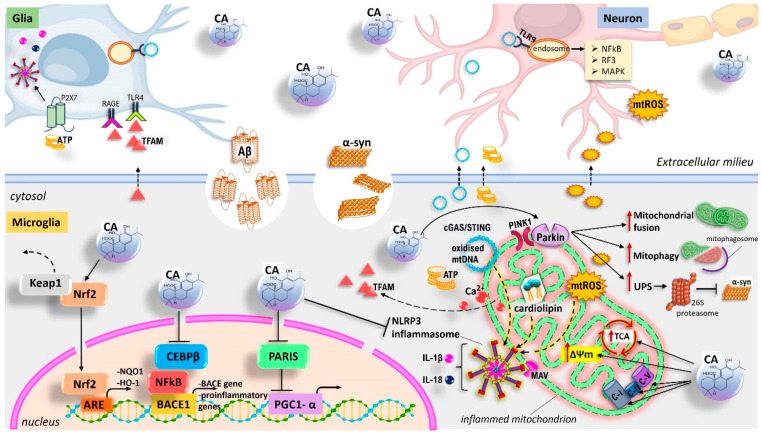
Figure 3.
Current model of multi-target protective effects of carnosic acid (CA) at the interface of mitochondrial dysfunction, neuroinflammation, and neurodegeneration. Upon stressful conditions, including oxidative injury, accumulation of altered/misfolded proteins, and inflammatory stimuli, mitochondria are impaired with ensuing release of mitochondrial-derived DAMPs (mtDAMPs) such as ROS, Ca2+, oxidized mtDNA, cardiolipin, ATP, and transcription factor A mitochondria (TFAM). In surrounding neurons and glial cells, mtDAMPs bind to TLR9, purinergic P2X7 receptor, RAGE, and TLR, promoting further mitochondrial damage and triggering pro-inflammatory, pro-apoptotic cascades that contribute to exacerbation of proinflammatory microglial activation, with the consequent degeneration of dopaminergic neurons. CA provides neuroprotective effects, decreasing oxidative stress by upregulating antioxidant defence, reducing the expression of proinflammatory cytokines via inhibiting the NFκB pathway and/or NLRP3 inflammasome activation, and preserving mitochondrial function. Therefore, Keap1 degradation and Nrf2/HO-1/NQO1 signalling pathway activation, upregulation of the PINK1/parkin pathway, and modulation of the PARIS/PGC-1α axis contribute to counteracting oxidative stress and energy imbalance, as well as mitochondrial disruption by promoting mitophagy, mitobiogenesis, mitochondrial fusion, and UPS machinery. Additionally, CA decreases the CEBPβ–NFκB interaction and reduces Aβ aggregation/deposition by inactivating the amyloidogenic proteolytic pathway of amyloid precursor protein (APP).