Abstract
Carotenoids have antioxidant properties, and the accumulation of advanced glycation end products (AGEs) is associated with reactive oxygen species production; they have attracted attention as factors predictive of the onset and progression in glaucoma. Fingertip measurement is applicable for carotenoids and AGEs due to its noninvasiveness and simplicity. The study included 663 eyes of 663 Japanese subjects (357 males, 306 females). The mean age was 69.9 years with a standard deviation of 11.0. The study population comprised participants with primary open-angle glaucoma (PG) (n = 358), exfoliation glaucoma (EG) (n = 168), and controls (n = 137). Multivariate models suggested that lower skin carotenoid (SC) levels were associated with male gender (standard β = −0.14), AGE scores (−0.24), and a history of intraocular surgery (−0.22). Higher SC levels were associated with higher vegetable intake scores (0.21 for score 3) and diabetes (0.10). However, no association was seen between SCs and glaucoma type. AGEs levels were negatively associated with carotenoid scores (−0.25), PG (−0.15), and smoking habits (−0.26) and positively correlated with EG (0.14). SCs and AGEs were negatively correlated in the single regression analysis (r = −0.20, p < 0.0001). In conclusion, higher levels of AGEs may be candidates for systemic biomarkers of glaucoma associated with the exfoliation syndrome. SC levels can reflect self-reported daily vegetable intake.
Keywords: skin carotenoid, fingertip sensor, Veggie Meter, advanced glycation end products (AGEs), AGE sensor, reactive oxygen species, oxidative stress, antioxidants, primary open-angle glaucoma, exfoliation glaucoma
1. Introduction
Glaucoma is a group of ophthalmic neurodegenerative diseases, and its progression is irreversible [1,2]. More than 70 million people in the world have glaucoma, making it the leading cause of low vision and blindness [3,4]. Elevated intraocular pressure (IOP) is a major risk factor, and lowering the IOP through medication or surgery is the main therapeutic option. Numerous risk factors, such as genetics, inflammation, ocular blood flow, and oxidative stress have been proposed by diverse nonclinical and clinical studies; however, the only reliable parameter used in clinical practice is IOP [5,6]. Elevated IOP levels are associated with retinal ganglion cell (RGC) death and inhibit blood flow, leading to oxidative stress [7,8,9]. Oxidative stress increases with the overproduction of reactive oxygen species (ROS) and dysfunction of the antioxidant system. Dysfunction of the mitochondria in glaucoma enhances ROS production and causes inflammatory injury of the RGCs [10,11]. Antioxidants suppress inflammation caused by ROS and improve RGCs in experimental glaucoma [12]. Neurodegeneration caused by ROS is considered a modifiable factor of glaucoma onset and progression [13,14]. ROS is mainly generated by the metabolism of the mitochondria and neutralized by antioxidants [15]. We previously reported that lower levels of serum biologic antioxidant potential were correlated with higher levels of IOP and with greater visual field loss in primary open-angle glaucoma (PG) [16,17,18].
Carotenoids, such as lutein and zeaxanthin, exist in the retina, especially in the fovea, and they are antioxidants [19,20,21,22]. These carotenoids scavenge free radicals to reduce oxidative stress and absorb visible light to inhibit the generation of light-induced ROS. Because humans cannot produce carotenoids in the body, they must be ingested [23]. The prevalence of glaucoma was lower in groups with high vegetable/fruit intake in epidemiologic studies [24,25]. Several clinical trials have been conducted to identify the neuroprotective benefits of carotenoids in glaucoma [26]. Macular pigment optical density (MPOD), which is the density of carotenoids, is lower in patients with glaucoma [27], and lutein supplementation increased carotenoid levels in the macular pigment [28,29,30,31]. Studies of dietary carotenoid supplementation in randomized controlled trials of glaucoma have been conducted; however, no significant effects were observed [32,33,34]. Even though carotenoids are supposed to play an important role in glaucoma, accurate monitoring of carotenoids is not yet possible. Percutaneous fingertip measurements may be suitable for monitoring carotenoids multiple times. These methods are noninvasive and convenient because they do not require blood sampling or difficult measurement procedures. The subjects place their fingers on the measurement device for only about 10 s for the measurement. The skin carotenoid (SC) levels obtained using the pressure-mediated reflection spectroscopy (RS) method were correlated with serum carotenoid levels measured by high-performance liquid chromatography (HPLC) [35]. The instrument can measure in the 350–850 nm range, which includes the absorption wavelengths of carotenoids around 480 nm. The association between skin melanin content and carotenoids is weakly correlated, indicating that SC levels are not affected by melanin absorption [35,36].
AGEs are generated by nonenzymatic glycation of proteins, nucleic acids, and lipids (Maillard reaction), followed by rearrangements and oxidative steps [37,38]. AGEs accumulate in ocular tissue and enhance ROS generation via the receptor for AGEs (RAGE). In the glaucomatous retina and optic nerve, the accumulation of AGEs is increased, and the expression of RAGE is upregulated. Upregulation of RAGE might cause early cell death of the RCGs [39]. AGEs levels were also measured percutaneously by the previously reported procedure using fingertip-measured skin autofluorescence (sAF) [40]. Among the AGEs, Nε-(carboxymethyl)-lysine (CML) was considered to be strongly involved in the pathogenic role, but it was difficult to detect directly because of the nonfluorescent feature of CML. sAF was correlated not only with fluorescent AGEs (Nδ-(5-hydro-5-methyl-4-imidazolone-2-yl)-ornithine, pentosidine, and collagen-linked fluorescence) but also with non-fluorescent AGEs (CML) [41,42]. This suggested that fluorescent and nonfluorescent AGEs had similar distributions, and that sAF can be used as a marker of AGE accumulation [41,42].
As part of our ongoing research program directed toward the analysis of the relationship between ophthalmologic disorders and stress markers measured by fingertip measurements [16,17,18], we investigated the clinical factors including SC and AGEs levels with glaucoma types. We previously reported AGEs levels in PG and EG [40]; however, the relationship between SC levels and glaucoma has not been reported. Herein, we report a comparison of the SC and AGEs levels in patients with and without glaucoma.
2. Materials and Methods
2.1. Subjects
The current study adhered to the tenets of the Declaration of Helsinki. The institutional review board (IRB) of Shimane University Hospital approved the research (No. 20200228-2, issued on 21 June 2021). The IRB approval did not require that each patient provide written informed consent for publication; instead, the study protocol was posted at the study institutions to opt the participants out of the study. Subjects were recruited consecutively at the Department of Ophthalmology, Shimane University Hospital from November 2019 to January 2021. This study included 663 eyes of 663 Japanese subjects in total (357 males, 306 females). The mean age was 69.9 years with a standard deviation of 11.0. The study population comprised participants with PG (n = 358), EG (n = 168), and controls (n = 137). Each participant underwent measurement of the best-corrected visual acuity (BCVA), Goldmann applanation tonometer-measured IOP, and slit-lamp and funduscopic examinations. The highest IOP recorded, the lens status, and the number of glaucoma medications used were collected from the previous medical charts. A combination medication was counted as two drugs. Information about the current smoking status, amount of vegetable intake, and history of diabetes and systemic hypertension was obtained during the medical interview. The amount of vegetable intake was estimated by the forced choice scale using a four-point rating system in which 0 indicated no or rarely, 1 indicated sometimes/small amount, 2 indicated frequent/sufficient amount, and 3 indicated very frequent/large amount. The diagnosis of PG was determined by the following observations: iridocorneal angles open in both eyes, distinctive appearance of glaucomatous optic neuropathy including the optic disc cup enlargement or the focal neuroretinal rim thinning, visual field defects corresponding to the optic disc appearance detected in at least in one eye, and no manifestation of secondary glaucoma seen bilaterally. The diagnosis of EG was determined on the basis of an open iridocorneal angle and distinctive pseudo-exfoliation material deposition on the lens capsule and/or pupillary margin in one or both eyes. When both eyes met the criteria, the eye with the worse visual field mean deviation (MD) was included in the PG or EG evaluation. Visual field defects were determined by the automatic visual field tester (Humphrey Visual Field Analyzer, Carl Zeiss Meditec, Dublin, CA, USA). The control subjects had no remarkable ocular disorders other than age-related cataracts, clinical findings of glaucoma, and glaucoma medication use. The highest IOP recorded was <21 mmHg in the control subjects. For controls, the eye with the better BCVA was included in the study. If the BCVA was the same in both eyes, the right eye was included. For all groups in this study, eyes with ocular pathologies other than glaucoma and age-related cataract were excluded. Patients with diabetic retinopathy were carefully excluded because a close association of AGEs with diabetes or its complications has been reported [43,44,45].
2.2. Measurement of Carotenoids in the Fingertip Skin
SC levels were measured by pressure-mediated RS (Veggie Meter®, Longevity Link Corporation, Salt Lake City, UT, USA). The Veggie Meter adopted pressure-mediated RS using a white light-emitting diode (350–850 nm) [35]. Experienced examiners performed all measurements. The measured scores were expressed as optical density (OD) units. The calibration was performed with the manufacturer-provided reference materials before the start of the morning and afternoon sessions. Participants inserted the left middle finger into the device’s finger cradle to measure the SC. The SC index was determined as the average of two consecutive measurements in 657 participants and by three measurements six 6 participants.
2.3. Measurement of AGEs in the Fingertip Skin
AGEs levels were measured by the AGEs Sensor (Air Water Biodesign Inc., Kobe, Japan). The sAF values were obtained at the excitation wavelength (365 nm) and emission wavelength (440 nm). Experienced examiners performed all measurements. The measured sAF was expressed as the AGE index in arbitrary units (AU). The AGE index was determined as the average of two consecutive measurements in 641 participants and by three measurements in 21 participants. For the triple AGE measurements, the mean coefficient of variation and Cronbach’s α intraclass correlation coefficient were 6.7% ± 7.3% and 0.938, respectively, in our pilot study.
2.4. Statistical Analysis
The data were expressed as numbers and percentages for categorical parameters, and as mean ± standard deviation (SD) with 95% confidence interval (CI) ranges for continuous parameters. The decimal BCVA was converted into the logarithm of the minimum angle of resolution (logMAR). Respective counting fingers, hand motions, light perception, and no light perception values were considered as the decimal visual acuities of 0.0025, 0.002, 0.0016, and 0.0013 [46]. For categorical parameters, group comparisons were performed using the G-test followed by Fisher’s exact probability test. For continuous parameters, group comparisons were performed by one-way analysis of variance (ANOVA) followed by unpaired t-tests. To correct for multigroup comparisons, using Bonferroni’s correlation, p-values of 0.0167 and 0.0033 were regarded as the significance levels of 5% and 1%, respectively, for the Fisher’s exact probability test or unpaired t-tests. Possible correlations among AGEs, SCs, and other parameters were calculated by the unpaired t-test for categorical variables, and by linear regression analyses with Pearson’s correlation coefficient for continuous variables. To correct for vegetable intake score, which has four categories, p-values of 0.0083 and 0.0016 for the Fisher’s exact probability test were regarded as the significance levels of 5% and 1%, respectively. We conducted further multiple regression analyses for possible associations among AGEs and SCs with assorted parameters to adjust differences among groups. JMP Pro statistical software version 16.1.0 (SAS Institute, Inc., Cary, NC, USA) was used for all statistical calculations in this study.
3. Results
The demographic characteristics of the patients, i.e., age, sex, BCVA, IOP, highest IOP, number of glaucoma medications, MD, lens status, current smoking status, diabetes, hypertension, vegetable intake scores, history of intraocular surgery, AGE scores, and SC scores, are shown in Table 1. Sex, current smoking status, diabetes, vegetable intake score, and carotenoids did not differ among the three groups, while the other parameters including AGE scores differed. The SC scores did not differ significantly among the three groups. However, the AGE scores of EG (0.48 ± 0.10) were significantly higher than those of the PG (0.44 ± 0.08, p < 0.0001) and control group (0.45 ± 0.08, p = 0.0012). No significant difference was seen between the PG and control group. The data underlying this article was described in Table S1.
Table 1.
Demographic subject data.
| Group | Control | PG | EG | p-Value a |
|---|---|---|---|---|
| 137 | 358 | 168 | ||
| Age (years) | ||||
| n | 137 | 358 | 168 | |
| Mean ± SD | 73.4 ± 10.9 | 66.1 ± 10.7 | 75.1 ± 8.6 | <0.0001 ** |
| 95% CI | 71.5 to 75.2 | 65.0 to 67.2 | 73.7 to 76.3 | |
| vs. control, p < 0.0001 b ## | vs. control, p = 0.1322 b | |||
| vs. PG, p < 0.0001 b ## | ||||
| Sex | ||||
| Male, n (%) | 64 (47) | 203 (57) | 90 (54) | 0.1364 |
| Female, n (%) | 73 (53) | 155 (43) | 78 (46) | |
| BCVA (logMAR) | ||||
| n | 137 | 358 | 168 | |
| Mean ± SD | 0.16 ± 0.22 | 0.19 ± 0.42 | 0.35 ± 0.61 | 0.0003 ** |
| 95% CI | 0.13 to 0.20 | 0.15 to 0.24 | 0.25 to 0.44 | |
| vs. control, p = 0.4680 b | vs. control, p = 0.0010 b ## | |||
| vs. PG, p = 0.0008 b ## | ||||
| IOP (mmHg) | ||||
| n | 90 | 357 | 168 | |
| Mean ± SD | 15.0 ± 2.7 | 15.4 ± 7.0 | 18.0 ± 9.2 | 0.0004 ** |
| 95% CI | 14.4 to 15.6 | 14.7 to 16.2 | 16.6 to 19.4 | |
| vs. control, p = 0.5504 b | vs. control, p = 0.0030 b ## | |||
| vs. PG, p = 0.0006 b ## | ||||
| Highest IOP (mmHg) | ||||
| 92 | 358 | 168 | ||
| Mean ± SD | 15.4 ± 3.5 | 21.4 ± 8.6 | 27.1 ± 11.4 | <0.0001 ** |
| 95% CI | 14.6 to 16.1 | 20.5 to 22.3 | 25.4 to 28.9 | |
| vs. control, p < 0.0001 b ## | vs. control, p < 0.0001 b ## | |||
| vs. PG, p < 0.0001 b ## | ||||
| No. of glaucoma medications | ||||
| n | 137 | 358 | 168 | |
| Mean ± SD | 0 | 2.5 ± 1.3 | 2.5 ± 1.4 | < 0.0001 ** |
| 95% CI | 2.4 to 2.7 | 2.3 to 2.7 | ||
| vs. control, p < 0.0001 b ## | vs. control, p < 0.0001b ## | |||
| vs. PG, p = 0.8741b | ||||
| MD (dB) | ||||
| n | 358 | 168 | ||
| Mean ± SD | – | −15.9 ± 8.4 | −18.0 ± 9.8 | 0.0114 ** |
| 95% CI | −16.8 to −15.0 | −19.4 to –16.7 | ||
| Pseudophakia | ||||
| Yes, n (%) | 19 (14) | 179 (50) | 119 (71) | <0.0001 ** |
| No, n (%) | 118 (86) | 179 (50) | 49 (29) | |
| vs. control, p < 0.0001 b ## | vs. control, p < 0.0001 b ## | |||
| vs. PG, p < 0.0001 b ## | ||||
| Current smoking | ||||
| Yes, n (%) | 15 (11) | 41 (12) | 18 (11) | 0.9586 |
| No, n (%) | 122 (89) | 315 (88) | 150 (89) | |
| Diabetes | ||||
| Yes, n (%) | 21 (21) | 53 (23) | 26 (20) | 0.8220 |
| No, n (%) | 78 (79) | 177 (77) | 102 (80) | |
| Hypertension | ||||
| Yes, n (%) | 48 (49) | 133 (55) | 85 (65) | 0.0449 * |
| No, n (%) | 49 (51) | 107 (45) | 45 (35) | |
| vs. control, p = 0.3366 b | vs. control, p = 0.0205 b | |||
| vs. PG, p = 0.0764 b | ||||
| Vegetable intake score | ||||
| n | 134 | 349 | 167 | |
| 0, n (%) | 11 (8) | 25 (7) | 16 (10) | 0.8103 |
| 1, n (%) | 23 (17) | 48 (14) | 28 (17) | |
| 2, n (%) | 72 (54) | 191 (55) | 89 (53) | |
| 3, n (%) | 28 (21) | 85 (24) | 34 (20) | |
| Intraocular surgery | ||||
| Yes, n (%) | 19 (14) | 223 (62) | 122 (73) | <0.0001 ** |
| No, n (%) | 118 (86) | 135 (38) | 46 (27) | |
| vs. control, p < 0.0001 b ## | vs. control, p < 0.0001 b ## | |||
| vs. PG, p = 0.0235 b | ||||
| SCs (OD) | ||||
| n | 137 | 358 | 168 | |
| Mean ± SD | 327.8 ± 125.7 | 336.2 ± 125.6 | 330.6 ± 114.0 | 0.7974 |
| 95% CI | 306.6 to 348.9 | 323.2 to 349.3 | 313.2 to 347.9 | |
| AGEs (AU) | ||||
| n | 137 | 358 | 168 | |
| Mean ± SD | 0.45 ± 0.08 | 0.44 ± 0.08 | 0.48 ± 0.10 | <0.0001 ** |
| 95% CI | 0.43 to 0.46 | 0.43 to 0.45 | 0.46 to 0.49 | |
| vs. control, p = 0.6818 b | vs. control, p = 0.0012 b ## | |||
| vs. PG, p < 0.0001 b ## |
a p-Values were estimated by ANOVA for continuous variables and by G-test for categorical variables. b Post hoc tests were performed by t-test or Fisher’s exact probability test. Significance levels at 5% (p < 0.05) *, 1% (p < 0.01) **, and 1% (p < 0.0033) ##. PG, primary open-angle glaucoma; EG, exfoliation glaucoma.
According to univariate analysis, no parameters of the continuous variables were correlated with the SC scores (Table 2). Male (p < 0.0001) and current smoking status (p < 0.0001) were associated with lower SC levels than their corresponding group (Table 3).
Table 2.
Possible associations among SCs and various continuous parameters.
| Parameters | r | Lower 95% CI | Upper 95% CI | p-Value |
|---|---|---|---|---|
| Age (years) | 0.03 | −0.05 | 0.10 | 0.4966 |
| BCVA (logMAR) | −0.06 | −0.13 | 0.02 | 0.1359 |
| IOP (mmHg) | 0.03 | −0.05 | 0.11 | 0.4243 |
| Highest IOP (mmHg) | −0.02 | −0.10 | 0.06 | 0.6725 |
| No. of glaucoma medications | 0.06 | −0.02 | 0.14 | 0.1200 |
Pearson’s correlation coefficient (r).
Table 3.
Possible association among SCs and various categorical parameters.
| Parameters | Mean ± SD (95% CI) | Mean ± SD (95% CI) | p-Value |
|---|---|---|---|
| Sex | Male, 310 ± 122 (297 to 323) | Female, 360 ± 118 (347 to 374) | <0.0001 ** |
| Pseudophakia | Yes, 332 ± 122 (319 to 346) | No, 334 ± 123 (321 to 347) | 0.8133 |
| Glaucoma type | PG, 336 ± 126 (323 to 349) | EG, 331 ± 114 (313 to 348) | 0.6210 |
| Intraocular surgery | Yes, 340 ± 123 (326 to 353) | No, 328 ± 123 (326 to 353) | 0.2360 |
| Current smoking | Yes, 252 ± 84 (232 to 271) | No, 344 ± 123 (333 to 354) | <0.0001 ** |
| Diabetes | Yes, 342 ± 136 (315 to 369) | No, 330 ± 119 (318 to 343) | 0.4285 |
| Hypertension | Yes, 325 ± 128 (309 to 340) | No, 341 ± 118 (325 to 358) | 0.1284 |
The p-values were estimated by t-test between groups. Significance level at 1% (p < 0.01) **.
Age (p < 0.0001) and BCVA (p < 0.0001) were positively correlated with AGE scores in the univariate regression analysis of continuous variables, whereas the IOP, highest IOP, and number of glaucoma medications were not correlated with AGE scores (Table 4). PG (p < 0.0001) and current smoking status (p < 0.0001) were associated with lower AGE levels than their corresponding group (Table 5).
Table 4.
Possible associations among AGEs and various continuous parameters.
| Parameters | r | Lower 95% CI | Upper 95% CI | p-Value |
|---|---|---|---|---|
| Age (year) | 0.17 | 0.10 | 0.24 | <0.0001 ** |
| BCVA (logMAR) | 0.15 | 0.08 | 0.23 | <0.0001 ** |
| IOP (mmHg) | 0.03 | −0.05 | 0.10 | 0.5333 |
| Highest IOP (mmHg) | 0.00 | −0.08 | 0.08 | 0.9929 |
| No. of glaucoma medications | 0.01 | −0.07 | 0.08 | 0.8753 |
Pearson’s correlation coefficient (r). Significance level at 1% (p < 0.01) **.
Table 5.
Possible association among AGEs and various categorical parameters.
| Parameters | Mean ± SD (95% CI) | Mean ± SD (95% CI) | p-Value |
|---|---|---|---|
| Sex | Male, 0.46 ± 0.09 (0.45 to 0.47) | Female, 0.45 ± 0.09 (0.44 to 0.46) | 0.1209 |
| Pseudophakia | Yes, 0.46 ± 0.09 (0.45 to 0.47) | No, 0.45 ± 0.08 (0.44 to 0.46) | 0.3183 |
| Glaucoma type | PG, 0.44 ± 0.08 (0.43 to 0.45) | EG, 0.48 ± 0.10 (0.46 to 0.49) | <0.0001 ** |
| Intraocular surgery | Yes, 0.46 ± 0.09 (0.45 to 0.47) | No, 0.45 ± 0.08 (0.44 to 0.46) | 0.2130 |
| Current smoking | Yes, 0.40 ± 0.09 (0.39 to 0.42) | No, 0.46 ± 0.08 (0.45 to 0.47) | <0.0001 ** |
| Diabetes | Yes, 0.46 ± 0.09 (0.45 to 0.48) | No, 0.45 ± 0.09 (0.44 to 0.46) | 0.2648 |
| Hypertension | Yes, 0.46 ± 0.09 (0.45 to 0.47) | No, 0.45 ± 0.08 (0.44 to 0.46) | 0.1284 |
The p-values were estimated by t-test between groups. Significance level at 1% (p < 0.01) **.
Table 6 shows the association between vegetable intake scores with AGEs scores and SC scores. SC scores differed depending on vegetable intake scores. The SC score of vegetable intake score 3 group (393 ± 124) was significantly higher than that of the score 0 (282 ± 112, p < 0.0001), 1 (267 ± 94, p < 0.0001), and 2 (323 ± 118, p < 0.0001) groups. The SC score of vegetable intake score 2 group was also significantly higher than that of the score 0 (p = 0.047) and 1 (p < 0.0001) groups. There was no significant difference in SC scores between vegetable intake score 1 and 0 groups. The AGE scores did not differ among the vegetable intake score groups.
Table 6.
Possible association between vegetable intake scores with SCs and AGEs.
| Vegetable Intake Score | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| n | 52 | 99 | 352 | 147 | |
| Parameters |
Mean ± SD
(95% CI) |
Mean ± SD
(95% CI) |
Mean ± SD
(95% CI) |
Mean ± SD
(95% CI) |
p-Value a |
| SCs (OD) | 282 ± 112 (255 to 317) |
267 ± 94 (249 to 286) |
335 ± 118 (323 to 347) |
393 ± 124 (373 to 413) |
<0.0001 ** |
| vs. 0, p = 0.1731 b | vs. 0, p = 0.0047 b # | vs. 0, p < 0.0001 b ## | |||
| vs. 1, p < 0.0001 b ## | vs. 1, p < 0.0001 b ## | ||||
| vs. 2, p < 0.0001 b ## | |||||
| AGEs (AU) | 0.47 ± 0.10 (0.45 to 0.50) |
0.45 ± 0.09 (0.44 to 0.47) |
0.45 ± 0.09 (0.45 to 0.46) |
0.44 ± 0.08 (0.43 to 0.46) |
0.1956 |
a p-Values were estimated by G-test. b Post hoc comparisons were performed by Fisher’s exact probability test. Significance levels at 1% (p < 0.01) **, 5% (p < 0.0083) #, and 1% (p < 0.0016) ##.
The multiple regression model analysis of SCs scores and various parameters is shown in Table 7. Male (female, standard β = −0.14, p = 0.0045), AGE scores (AU, standard β = −0.24, p < 0.0001), and a history of intraocular surgery (no, standard β = −0.22, p = 0.0359) were negatively correlated with lower SC scores, while diabetes (no, standard β = 0.10, p = 0.0376) was positively correlated with higher SC scores (Table 7). A vegetable intake score of 3 was correlated with higher SC scores (0, standard β = 0.21, p < 0.0001); however, the vegetable intake score 1 was correlated with lower SC scores (0, standard β = −0.16, p = 0.0014).
Table 7.
Possible associations among SCs and various parameters analyzed by multiple regression analysis.
| Parameters | Estimate | Lower 95% CI | Upper 95% CI | p Value | Standard β |
|---|---|---|---|---|---|
| Age (year) | 0.64 | −0.62 | 1.91 | 0.3200 | 0.06 |
| Male (female) | −16.98 | −28.65 | −5.30 | 0.0045 ** | −0.14 |
| BCVA (logMAR) | −7.91 | −33.16 | 17.33 | 0.5380 | −0.03 |
| IOP (mmHg) | −0.56 | −2.63 | 1.52 | 0.5984 | −0.04 |
| Highest IOP (mmHg) | 0.80 | −1.12 | 2.72 | 0.4121 | 0.06 |
| No. of glaucoma medications | 6.70 | −2.96 | 16.38 | 0.1733 | 0.09 |
| AGEs (AU) | −346.64 | −484.02 | −209.26 | <0.0001 ** | −0.24 |
| Phakia (pseudophakia) | −17.92 | −43.48 | 7.63 | 0.1686 | −0.14 |
| Intraocular surgery, yes (no) | −27.17 | −52.54 | −1.79 | 0.0359 * | −0.22 |
| PG (control) | −4.08 | −23.08 | 14.93 | 0.6734 | −0.02 |
| EG (control) | 9.98 | −11.09 | 31.05 | 0.3524 | 0.05 |
| Current smoking, yes (no) | −41.72 | −61.15 | −22.30 | <0.0001 ** | −0.21 |
| Diabetes, yes (no) | 15.79 | 0.91 | 30.67 | 0.0376 * | 0.10 |
| Hypertension, yes (no) | −7.97 | −19.73 | 3.79 | 0.1837 | −0.06 |
| Vegetable intake score 1 (0) | −41.04 | −66.06 | −16.03 | 0.0014 ** | −0.16 |
| Vegetable intake score 2 (0) | 10.60 | −7.17 | 28.36 | 0.2415 | 0.05 |
| Vegetable intake score 3 (0) | 48.79 | 26.75 | 70.83 | <0.0001 ** | 0.21 |
p-Values were estimated by a multiple regression model. Significance levels at 5% (p < 0.05) * and 1% (p < 0.01) **.
The multiple regression model analysis of the AGE scores and various parameters is shown in Table 8. SC scores (OD, standard β = −0.25, p < 0.0001), PG (control, standard β = −0.15, p = 0.0112), and smoking status (no, standard β = −0.26, p < 0.0001) were negatively correlated with the AGE scores level, while EG (control, standard β = 0.14, p = 0.0173) was positively correlated with the AGE scores level.
Table 8.
Possible associations among AGEs and various parameters analyzed by multiple regression analysis.
| Parameters | Estimate | Lower 95% CI | Upper 95% CI | p-Value | Standard β |
|---|---|---|---|---|---|
| Age (year) | 0.001 | −0.00009 | 0.002 | 0.0784 | 0.10 |
| Male (female) | 0.078 | −0.0005 | 0.016 | 0.0661 | 0.09 |
| BCVA (logMAR) | 0.003 | −0.015 | 0.021 | 0.7230 | 0.02 |
| IOP (mmHg) | −0.002 | −0.002 | 0.013 | 0.8295 | −0.02 |
| Highest IOP (mmHg) | −0.003 | −0.002 | 0.001 | 0.6913 | −0.03 |
| No. of glaucoma medications | 0.057 | −0.001 | 0.013 | 0.1040 | 0.10 |
| Phakia (pseudophakia) | 0.019 | 0.0008 | 0.037 | 0.2203 | 0.22 |
| Intraocular surgery, yes (no) | 0.014 | −0.004 | 0.032 | 0.1315 | 0.16 |
| SCs (OD) | −0.0002 | −0.0002 | −0.001 | <0.0001 ** | −0.25 |
| PG (control) | −0.018 | −0.031 | −0.004 | 0.0112 * | −0.15 |
| EG (control) | 0.018 | 0.003 | 0.033 | 0.0173 * | 0.14 |
| Current smoking, yes (no) | −0.036 | −0.050 | −0.022 | <0.0001 ** | −0.26 |
| Diabetes, yes (no) | 0.008 | −0.002 | 0.019 | 0.1237 | 0.08 |
| Hypertension, yes (no) | 0.004 | −0.005 | 0.012 | 0.4099 | 0.04 |
| Vegetable intake score 1 (0) | −0.006 | −0.024 | 0.012 | 0.5120 | −0.03 |
| Vegetable intake score 2 (0) | −0.008 | −0.021 | 0.005 | 0.2181 | −0.06 |
| Vegetable intake score 3 (0) | −0.005 | −0.021 | 0.012 | 0.5814 | −0.03 |
p-Values were estimated by a multiple regression model. Significance levels at 5% (p < 0.05) * and 1% (p < 0.01) **.
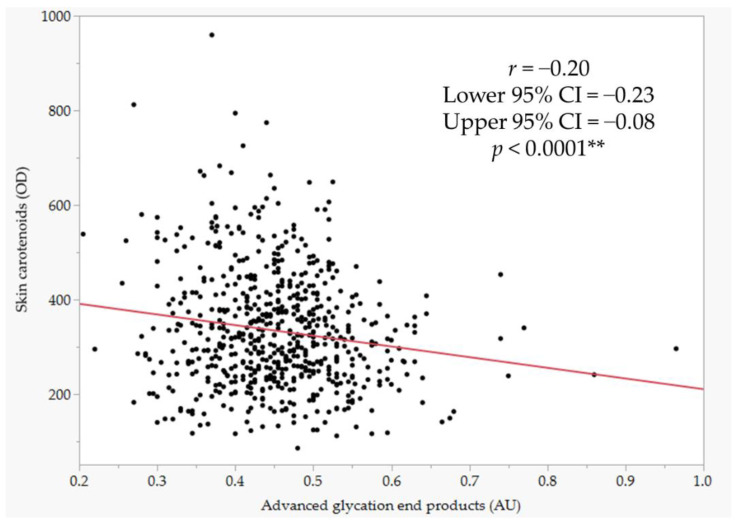
The scatterplot in Figure 1 shows the correlation between AGE scores and SC scores. According to regression analysis, they were significantly correlated with each other. The correlation coefficient (r) was −0.20, and the p-value was less than 0.0001.
Figure 1.
Single regression of AGEs and SCs. The p-value was estimated by a linear regression analysis. Significance level at 1% (p < 0.01) **.
4. Discussion
To the best of our knowledge, this study is the first to simultaneously estimate the AGE and SC levels using fingertip sensors in patients with glaucoma. This study found that low SC levels were associated with male gender, history of intraocular surgery, current smoking status, diabetes, low vegetable intake score, and high levels of AGEs by multiple regression analysis; however, no significant association with glaucoma type was detected. On the other hand, AGEs levels were higher in EG than PG and controls. In addition, a negative association was found between SC and AGEs levels.
SC levels measured by the Veggie Meter® were strongly correlated with serum carotenoid levels detected by HPLC [35]. The meter, using pressure-mediated RS, detects skin chromophores between 400 and 750 nm. Most carotenoids, such as α- and β-carotenes, β-cryptoxanthin, lycopene, lutein, and zeaxanthin, have a maximal absorption wavelength of around 480 nm; therefore, the carotenoid score reflects the bulk of these carotenoid molecules. Intraocular levels of lutein, (3R,3′R)-zeaxanthin, and meso-(3R,3′S)-zeaxanthin, the only carotenoids present in macular pigment [22], and their antioxidant activity are difficult to estimate directly. Although the carotenoid levels reflect previous intake of vegetables, this lifestyle factor is also difficult to determine. Given that SC levels were mainly associated with vegetable intake for about 1 month [47], SC can be a good endpoint to assess the roles of carotenoids in various diseases in clinical situations. In this study, the role of SCs in the differentiation of glaucoma types or the suitability of SCs in glaucoma management was not determined. Ophthalmic neurodegeneration in glaucoma occurs over several decades [1,4]; therefore, the risk factors for disease onset and progression should be affected by time course. Fluctuation in each patient’s vegetable intake over a long period of years would be one explanation for the absence of a correlation between SC and glaucoma. Because of the bulked estimation of various carotenoids, the roles of specific carotenoid molecules might be masked in our methodology. Several factors were correlated with SCs by multiple regression analysis (Table 7). The results for smoking status [48] and gender [23] were consistent with previous reports, suggesting the proper estimation of carotenoid levels by this study. Several epidemiologic studies reported that the group with higher fruit/vegetable intake had a lower prevalence of glaucoma [24]. Administration of carotenoid-containing supplements to healthy subjects increased carotenoid levels in serum and MPOD [29,31,49]. Serum levels of carotenoids have not been evaluated in previous epidemiologic and carotenoid administration studies in glaucoma, although MPOD has been measured [26]. The literature did not contain any relevant previous studies that assessed the association between SC levels and the type of glaucoma (PG, EG). Fingertip-measured carotenoid levels would be beneficial for use in future studies due to its compatibility.
Significant differences in AGEs were observed by glaucoma type, with higher AGEs in EG and lower AGEs in PG (Table 8). The result of AGEs in EG was consistent with our previous report [40]. Exfoliation products are produced by the aggregation of microfibrils [50], and the effect of AGEs via RAGE may enhance the production. Tezel et al. reported that AGE deposition and RAGE upregulation were observed in the retina and optic nerve head in glaucoma. The RAGE upregulation in RGCs and glia might be involved in early optic nerve degeneration [39]. Since the production of AGEs enhanced by ROS was irreversible and this accumulation proceeded over years, short-term fluctuations were small; therefore, AGEs measured by fingertip would reflect AGEs in the eye [37]. The reason why AGEs were lower in PG was unclear but suggested that the background factors of the disease differed from those of EG. SCs and AGEs were negatively correlated with each other (Figure 1). Dietary intake of carotenoids would be involved in modifying AGE accumulation in the body. The formation process of AGEs includes oxidative reactions [38]; therefore, the antioxidant effect of carotenoids suppressed these oxidation steps to reduce the production of AGEs accordingly [13]. This was supported by a report that administration of chestnut (Tarpa bispinosa Roxb.) extract and lutein decreased the AGEs levels [51].
The current study had several limitations. The subject demographic data (Table 1) differed significantly in age, BCVA, IOP, highest IOP, number of glaucoma medications, MD, phakia status, hypertension, and history of intraocular surgery. These factors might have affected the results, although we attempted to minimize the effect using multivariate analyses (Table 7 and Table 8). Current smoking habits, diabetes, hypertension, and vegetable intake scores were collected by interviews, which might lower the power of detection. Although the vegetable intake score was not obtained using a standard questionnaire [52], our method would be beneficial because it was correlated with SC levels. Since the Veggie Meter® detects carotenoids as mixtures, it might not be able to extract the specific carotenoids associated with glaucoma pathotypes. Long-term measurements and more accurate methods are desirable to elucidate the relationship between carotenoid levels measured by fingertip and glaucoma. Given its easy-to-measure nature, estimation of both SCs and AGEs by fingertip can be applicable for exploring the biomarkers of various pathologies other than eye diseases.
5. Conclusions
In conclusion, this study did not detect an association between SC levels and glaucoma types, although SC levels were associated with vegetable intake scores. Furthermore, higher levels of AGEs may be useful candidates as systemic biomarkers of glaucoma associated with exfoliation syndrome; AGEs might be useful to distinguish two types of open-angle glaucoma.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11061138/s1, Table S1: Data underlying the manuscript.
Author Contributions
Conceptualization, Y.K., Y.T., J.S. and M.T.; methodology, M.T.; formal analysis, Y.K., Y.T., J.S. and M.T.; investigation, Y.K., Y.T., J.S. and M.T.; data curation, Y.K., Y.T., J.S. and M.T.; writing—original draft preparation, Y.K. and M.T.; writing—review and editing, Y.T. and J.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The current study adhered to the tenets of the Declaration of Helsinki. The institutional review board of Shimane University Hospital approved the research (No. 20200228-2, issued on 21 June 2021).
Informed Consent Statement
The institutional review board (IRB) approval did not require that each patient provide written informed consent for publication; instead, the study protocol was posted at the study institutions to opt the participants out of the study.
Data Availability Statement
The data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weinreb R.N., Khaw P.T. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Jonas J.B., Aung T., Bourne R.R., Bron A.M., Ritch R., Panda-Jonas S. Glaucoma. Lancet. 2017;390:2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 3.Foster A., Resnikoff S. The impact of Vision 2020 on global blindness. Eye. 2005;19:1133–1135. doi: 10.1038/sj.eye.6701973. [DOI] [PubMed] [Google Scholar]
- 4.Iwase A., Suzuki Y., Araie M., Yamamoto T., Abe H., Shirato S., Kuwayama Y., Mishima H.K., Shimizu H., Tomita G., et al. The prevalence of primary open-angle glaucoma in Japanese: The Tajimi Study. Ophthalmology. 2004;111:1641–1648. doi: 10.1016/S0161-6420(04)00665-7. [DOI] [PubMed] [Google Scholar]
- 5.Lim R. The surgical management of glaucoma: A review. Clin. Exp. Ophthalmol. 2022;50:213–231. doi: 10.1111/ceo.14028. [DOI] [PubMed] [Google Scholar]
- 6.Casson R.J. Medical therapy for glaucoma: A review. Clin. Exp. Ophthalmol. 2022;50:198–212. doi: 10.1111/ceo.13989. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarieh M., Grieshaber M.C., Flammer J. Oxygen and blood flow: Players in the pathogenesis of glaucoma. Mol. Vis. 2008;14:224–233. [PMC free article] [PubMed] [Google Scholar]
- 8.Vernazza S., Oddone F., Tirendi S., Bassi A.M. Risk Factors for Retinal Ganglion Cell Distress in Glaucoma and Neuroprotective Potential Intervention. Int. J. Mol. Sci. 2021;22:7994. doi: 10.3390/ijms22157994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinazo-Duran M.D., Shoaie-Nia K., Zanon-Moreno V., Sanz-Gonzalez S.M., Del Castillo J.B., Garcia-Medina J.J. Strategies to Reduce Oxidative Stress in Glaucoma Patients. Curr. Neuropharmacol. 2018;16:903–918. doi: 10.2174/1570159X15666170705101910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chrysostomou V., Rezania F., Trounce I.A., Crowston J.G. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharmacol. 2013;13:12–15. doi: 10.1016/j.coph.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Tezel G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog. Retin. Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Hondur G., Tezel G. Antioxidant Treatment Limits Neuroinflammation in Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2016;57:2344–2354. doi: 10.1167/iovs.16-19153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dziedziak J., Kasarello K., Cudnoch-Jedrzejewska A. Dietary Antioxidants in Age-Related Macular Degeneration and Glaucoma. Antioxidants. 2021;10:1743. doi: 10.3390/antiox10111743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tezel G. Molecular regulation of neuroinflammation in glaucoma: Current knowledge and the ongoing search for new treatment targets. Prog. Retin. Eye. Res. 2022;87:100998. doi: 10.1016/j.preteyeres.2021.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang E.Y., Liu P.K., Wen Y.T., Quinn P.M.J., Levi S.R., Wang N.K., Tsai R.K. Role of Oxidative Stress in Ocular Diseases Associated with Retinal Ganglion Cells Degeneration. Antioxidants. 2021;10:1948. doi: 10.3390/antiox10121948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanito M., Kaidzu S., Takai Y., Ohira A. Correlation between Systemic Oxidative Stress and Intraocular Pressure Level. PLoS ONE. 2015;10:e0133582. doi: 10.1371/journal.pone.0133582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanito M., Kaidzu S., Takai Y., Ohira A. Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Sci. Rep. 2016;6:25792. doi: 10.1038/srep25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takayanagi Y., Takai Y., Kaidzu S., Tanito M. Evaluation of Redox Profiles of the Serum and Aqueous Humor in Patients with Primary Open-Angle Glaucoma and Exfoliation Glaucoma. Antioxidants. 2020;9:1305. doi: 10.3390/antiox9121305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiedor J., Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obana A., Gohto Y., Asaoka R., Gellermann W., Bernstein P.S. Lutein and Zeaxanthin Distribution in the Healthy Macula and Its Association with Various Demographic Factors Examined in Pseudophakic Eyes. Antioxidants. 2021;10:1857. doi: 10.3390/antiox10121857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khachik F., Bernstein P.S., Garland D.L. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Investig. Ophthalmol. Vis. Sci. 1997;38:1802–1811. [PubMed] [Google Scholar]
- 22.Krinsky N.I., Landrum J.T., Bone R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 23.Bohm V., Lietz G., Olmedilla-Alonso B., Phelan D., Reboul E., Banati D., Borel P., Corte-Real J., de Lera A.R., Desmarchelier C., et al. From carotenoid intake to carotenoid blood and tissue concentrations-implications for dietary intake recommendations. Nutr. Rev. 2021;79:544–573. doi: 10.1093/nutrit/nuaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giaconi J.A., Yu F., Stone K.L., Pedula K.L., Ensrud K.E., Cauley J.A., Hochberg M.C., Coleman A.L., Study of Osteoporotic Fractures Research G. The association of consumption of fruits/vegetables with decreased risk of glaucoma among older African-American women in the study of osteoporotic fractures. Am. J. Ophthalmol. 2012;154:635–644. doi: 10.1016/j.ajo.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang J.H., Pasquale L.R., Willett W., Rosner B., Egan K.M., Faberowski N., Hankinson S.E. Antioxidant intake and primary open-angle glaucoma: A prospective study. Am. J. Epidemiol. 2003;158:337–346. doi: 10.1093/aje/kwg167. [DOI] [PubMed] [Google Scholar]
- 26.Lem D.W., Gierhart D.L., Davey P.G. Carotenoids in the Management of Glaucoma: A Systematic Review of the Evidence. Nutrients. 2021;13:1949. doi: 10.3390/nu13061949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siah W.F., O’Brien C., Loughman J.J. Macular pigment is associated with glare-affected visual function and central visual field loss in glaucoma. Br. J. Ophthalmol. 2018;102:929–935. doi: 10.1136/bjophthalmol-2017-310215. [DOI] [PubMed] [Google Scholar]
- 28.Obana A., Gohto Y., Nakazawa R., Moriyama T., Gellermann W., Bernstein P.S. Effect of an antioxidant supplement containing high dose lutein and zeaxanthin on macular pigment and skin carotenoid levels. Sci. Rep. 2020;10:10262. doi: 10.1038/s41598-020-66962-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshizako H., Hara K., Takai Y., Kaidzu S., Obana A., Ohira A. Comparison of macular pigment and serum lutein concentration changes between free lutein and lutein esters supplements in Japanese subjects. Acta Ophthalmol. 2016;94:e411–e416. doi: 10.1111/aos.13106. [DOI] [PubMed] [Google Scholar]
- 30.Obana A., Tanito M., Gohto Y., Okazaki S., Gellermann W., Bernstein P.S. Changes in Macular Pigment Optical Density and Serum Lutein Concentration in Japanese Subjects Taking Two Different Lutein Supplements. PLoS ONE. 2015;10:e0139257. doi: 10.1371/journal.pone.0139257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanito M., Obana A., Gohto Y., Okazaki S., Gellermann W., Ohira A. Macular pigment density changes in Japanese individuals supplemented with lutein or zeaxanthin: Quantification via resonance Raman spectrophotometry and autofluorescence imaging. Jpn. J. Ophthalmol. 2012;56:488–496. doi: 10.1007/s10384-012-0157-0. [DOI] [PubMed] [Google Scholar]
- 32.Sanz-González S.M., Raga-Cervera J., Aguirre Lipperheide M., Zanón-Moreno V., Chiner V., Ramírez A.I., Pinazo-Durán M.D. Effect of an oral supplementation with a formula containing R-lipoic acid in glaucoma patients. Arch. De La Soc. Esp. De Oftalmol. (Engl. Ed.) 2020;95:120–129. doi: 10.1016/j.oftal.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Medina J.J., Garcia-Medina M., Garrido-Fernandez P., Galvan-Espinosa J., Garcia-Maturana C., Zanon-Moreno V., Pinazo-Duran M.D. A two-year follow-up of oral antioxidant supplementation in primary open-angle glaucoma: An open-label, randomized, controlled trial. Acta Ophthalmol. 2015;93:546–554. doi: 10.1111/aos.12629. [DOI] [PubMed] [Google Scholar]
- 34.Romeo Villadoniga S., Rodriguez Garcia E., Sagastagoia Epelde O., Alvarez Diaz M.D., Domingo Pedrol J.C. Effects of Oral Supplementation with Docosahexaenoic Acid (DHA) plus Antioxidants in Pseudoexfoliative Glaucoma: A 6-Month Open-Label Randomized Trial. J. Ophthalmol. 2018;2018:8259371. doi: 10.1155/2018/8259371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ermakov I.V., Ermakova M., Sharifzadeh M., Gorusupudi A., Farnsworth K., Bernstein P.S., Stookey J., Evans J., Arana T., Tao-Lew L., et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch. Biochem. Biophys. 2018;646:46–54. doi: 10.1016/j.abb.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takayanagi Y., Obana A., Muto S., Asaoka R., Tanito M., Ermakov I.V., Bernstein P.S., Gellermann W. Relationships between Skin Carotenoid Levels and Metabolic Syndrome. Antioxidants. 2021;11:14. doi: 10.3390/antiox11010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kandarakis S.A., Piperi C., Topouzis F., Papavassiliou A.G. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Prog. Retin. Eye Res. 2014;42:85–102. doi: 10.1016/j.preteyeres.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Thornalley P.J., Langborg A., Minhas H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Pt 1Biochem. J. 1999;344:109–116. doi: 10.1042/bj3440109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tezel G., Luo C., Yang X. Accelerated aging in glaucoma: Immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Investig. Ophthalmol. Vis. Sci. 2007;48:1201–1211. doi: 10.1167/iovs.06-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirakami T., Yamanaka M., Fujihara J., Matsuoka Y., Gohto Y., Obana A., Tanito M. Advanced Glycation End Product Accumulation in Subjects with Open-Angle Glaucoma with and without Exfoliation. Antioxidants. 2020;9:755. doi: 10.3390/antiox9080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamanaka M., Matsumura T., Ohno R., Fujiwara Y., Shinagawa M., Sugawa H., Hatano K., Shirakawa J., Kinoshita H., Ito K., et al. Non-invasive measurement of skin autofluorescence to evaluate diabetic complications. J. Clin. Biochem. Nutr. 2016;58:135–140. doi: 10.3164/jcbn.15-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meerwaldt R., Graaff R., Oomen P.H.N., Links T.P., Jager J.J., Alderson N.L., Thorpe S.R., Baynes J.W., Gans R.O.B., Smit A.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47:1324–1330. doi: 10.1007/s00125-004-1451-2. [DOI] [PubMed] [Google Scholar]
- 43.Bentata R., Cougnard-Grégoire A., Delyfer M.N., Delcourt C., Blanco L., Pupier E., Rougier M.B., Rajaobelina K., Hugo M., Korobelnik J.F., et al. Skin autofluorescence, renal insufficiency and retinopathy in patients with type 2 diabetes. J. Diabetes Its Complicat. 2017;31:619–623. doi: 10.1016/j.jdiacomp.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 44.Yasuda M., Shimura M., Kunikata H., Kanazawa H., Yasuda K., Tanaka Y., Konno H., Takahashi M., Kokubun T., Maruyama K., et al. Relationship of skin autofluorescence to severity of retinopathy in type 2 diabetes. Curr. Eye Res. 2015;40:338–345. doi: 10.3109/02713683.2014.918152. [DOI] [PubMed] [Google Scholar]
- 45.Gerrits E.G., Lutgers H.L., Kleefstra N., Graaff R., Groenier K.H., Smit A.J., Gans R.O., Bilo H.J. Skin autofluorescence: A tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care. 2008;31:517–521. doi: 10.2337/dc07-1755. [DOI] [PubMed] [Google Scholar]
- 46.Grover S., Fishman G.A., Anderson R.J., Tozatti M.S.V., Heckenlively J.R., Weleber R.G., Edwards A.O., Brown J. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology. 1999;106:1780–1785. doi: 10.1016/S0161-6420(99)90342-1. [DOI] [PubMed] [Google Scholar]
- 47.Ermakov I.V., Gellermann W. Dermal carotenoid measurements via pressure mediated reflection spectroscopy. J. Biophotonics. 2012;5:559–570. doi: 10.1002/jbio.201100122. [DOI] [PubMed] [Google Scholar]
- 48.Sugiura M., Nakamura M., Ogawa K., Ikoma Y., Matsumoto H., Ando F., Shimokata H., Yano M. Synergistic interaction of cigarette smoking and alcohol drinking with serum carotenoid concentrations: Findings from a middle-aged Japanese population. Br. J. Nutr. 2009;102:1211–1219. doi: 10.1017/S0007114509382124. [DOI] [PubMed] [Google Scholar]
- 49.Bone R.A., Davey P.G., Roman B.O., Evans D.W. Efficacy of Commercially Available Nutritional Supplements: Analysis of Serum Uptake, Macular Pigment Optical Density and Visual Functional Response. Nutrients. 2020;12:1321. doi: 10.3390/nu12051321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlotzer-Schrehardt U., Khor C.C. Pseudoexfoliation syndrome and glaucoma: From genes to disease mechanisms. Curr. Opin. Ophthalmol. 2021;32:118–128. doi: 10.1097/ICU.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 51.Jinno M., Nagai R., Takeuchi M., Watanabe A., Teruya K., Sugawa H., Hatakeyama N., Jinno Y. Trapa bispinosa Roxb. extract lowers advanced glycation end-products and increases live births in older patients with assisted reproductive technology: A randomized controlled trial. Reprod. Biol. Endocrinol. 2021;19:149. doi: 10.1186/s12958-021-00832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Block G., Hartman A.M., Dresser C.M., Carroll M.D., Gannon J., Gardner L. A data-based approach to diet questionnaire design and testing. Am. J. Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are contained within the article and Supplementary Materials.