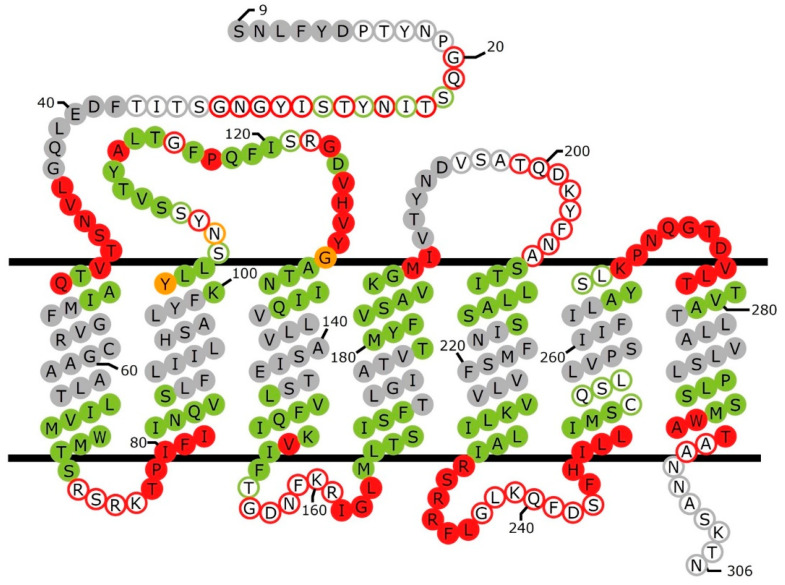
Figure 1.
Schematic view of accessibility measurements and helical segments in Ste2p. Filled circles of different colors indicate positions of residues that map to α-helical regions of the ligand-free structure (PDB: 7QB9) [37]. Circles of different colors with white backgrounds show the positions of residues that do not reside in α-helical segments. Positions that, when replaced by cysteine, exhibited greater than 50% reactivity with MTSEA-biotin, compared to controls presented in the corresponding publications [43,44,45,46,48], are shown in red. Positions where cysteine substitutions were tested but exhibited less than 50% reactivity with MTSEA-biotin are shown in green. Positions that were not tested for cysteine accessibility are shown in grey. Positions labeled in orange were reported to have different accessibilities in different publications. Approximate boundaries of the membrane region are indicated. Positions of amino acids in the figure are not intended to correspond to their actual locations in the cryo-EM structure. The first eight residues of the N terminus and the last ~125 residues at the C terminus of Ste2p were not resolved in the structure, and are not included in the figure.