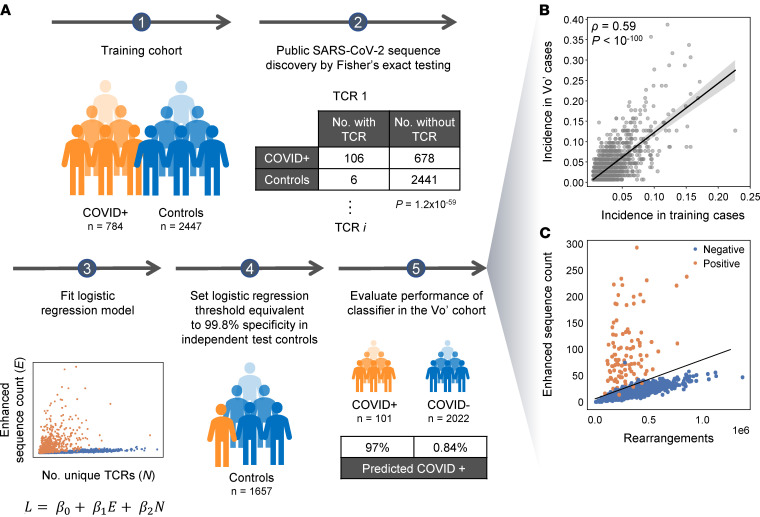
Figure 1. Identification and use of public T cell receptors to characterize the SARS-CoV-2 immune response.
(A) Schema of the previously generated classification framework, also described in refs. 18 and 33, starting from a case-control design and Fisher’s exact testing for each TCR on independent training data, to identify public TCR sequences that are overrepresented in cases versus controls. Following logistic regression to establish the T cell test threshold for determining recent or past infection, the receptors are applied to this Vo’ study data set. COVID+ samples include all 101 samples defined by the positive ground truth samples set from Dorigatti et al. (36), while controls included the 2022 samples that were negative by PCR and all 3 serology tests. (B) Incidence of each TCR sequence compared in the training data and in the Vo’ PCR+ cases. (C) The count of enhanced sequences is plotted versus the total number of unique TCR rearrangements for individuals in the Vo’ study data set that were positive (orange) or negative (blue).