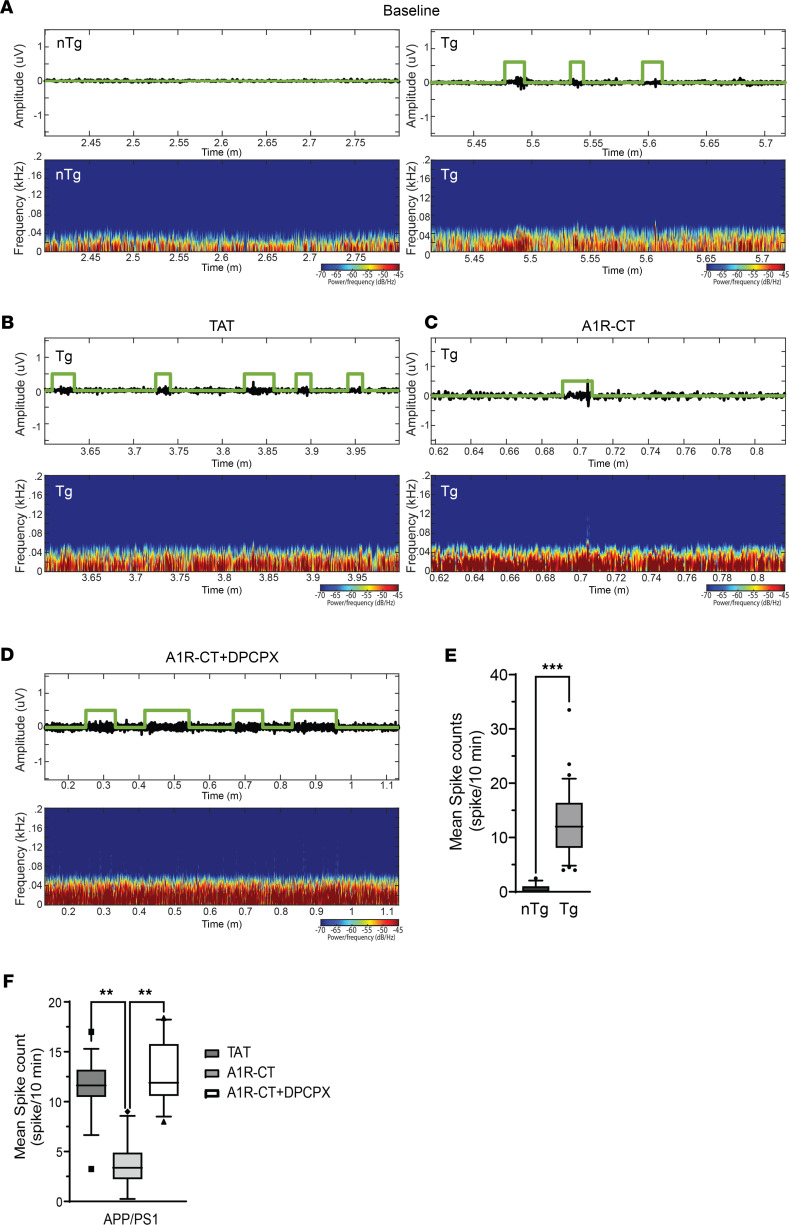
Figure 7. Intranasal delivery of the A1R-CT peptide effectively reduces epileptic activities in the brain of APP/PS1 mice.
Eleven-month-old APP/PS1 and nTg littermates were subjected to EEG recording. (A) Representative EEG trace and spectrogram in nTg and APP/PS1 mice at baseline. (B–D) Representative EEG trace and spectrogram in APP/PS1 mice after TAT (B) or TAT-fused A1R-CT (C) peptide treatment or A1R-CT+DPCPX treatment (D). (E) Quantitation of spike frequency with repeated measurement in nTg and APP/PS1 mice at baseline. Data were obtained from analyzing EEG recordings of 3 nTg and 3 APP/PS1 mice. (F) Quantitation of spike frequency with repeated measurement in APP/PS1 mice with indicated treatments. Data were obtained from analyzing EEG recordings of 4 TAT-treated, 4 A1R-CT–treated and 5 A1R-CT+DPCPX–treated APP/PS1 mice. Box-and-whisker plots represent median and 5–95 percentile range of all measurements for each group. **P < 0.01, ***P < 0.001 by a repeated measure 1-way ANOVA used to calculate the statistical difference of the spike count per 10 minutes between nTg and APP/PS1 mice and among different treatments in APP/PS1 mice.