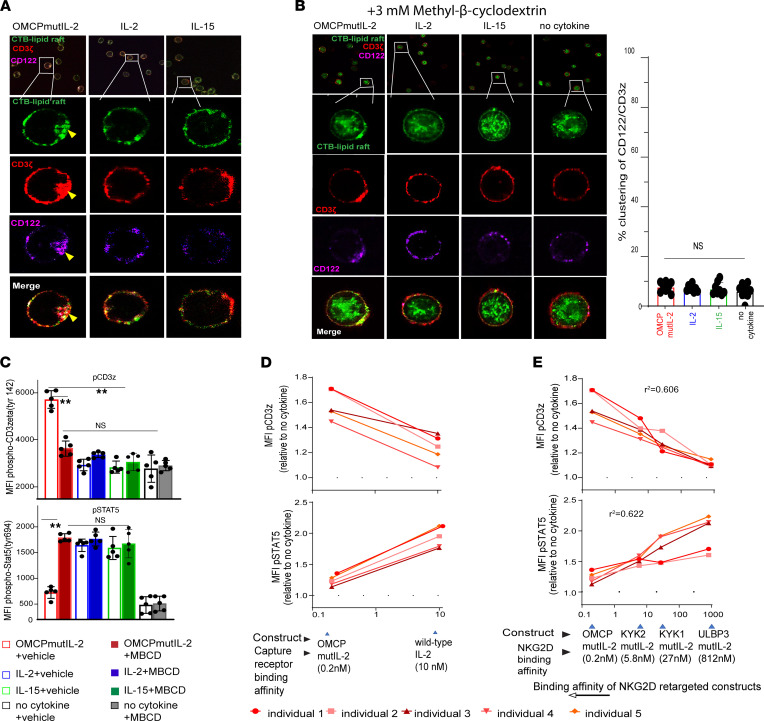
Figure 7. OMCPmutIL-2–mediated reorganization of surface membrane receptors is lipid raft dependent, and downstream TCR phosphorylation depends on cytokine binding affinity.
(A) High-resolution confocal microscopy of human CD8+ T cells evaluating receptor localization/clustering of CD3ζ (red) and IL-2Rβ/CD122 (pink) with lipid rafts (stained by Cholera Toxin B, CTB, in green). Clustering of receptors is shown by yellow arrow. (B) High-resolution confocal microscopy of human CD8+ T cell surface receptors (left panel) IL-2Rβ (CD122) (green), CD3ζ (red), and lipid rafts (stained by CTB in green) after lipid raft disruption by Methyl-β-cyclodextrin (MBCD) demonstrates no visible colocalization of receptors. Percentage clustering (right panel) shows little clustering and no difference between groups after MBCD treatment. Original magnification, ×63. (C) Flow cytometric analysis of CD3ζ and STAT5 phosphorylation after 1 hour of stimulation in various cytokines in the presence or absence of MBCD. (D) Phospho-CD3ζ and phospho-STAT5 expression in human PBL-derived CD8+ T cells after culture with OMCPmutIL-2 versus IL-2. (E) Phospho-CD3ζ and phospho-STAT5 expression in human PBL-derived CD8+ T cells after culture with mutIL-2–redirected constructs with different NKG2D ligands having different binding affinity. **P < 0.01; t test (B–D) or ANOVA (E).