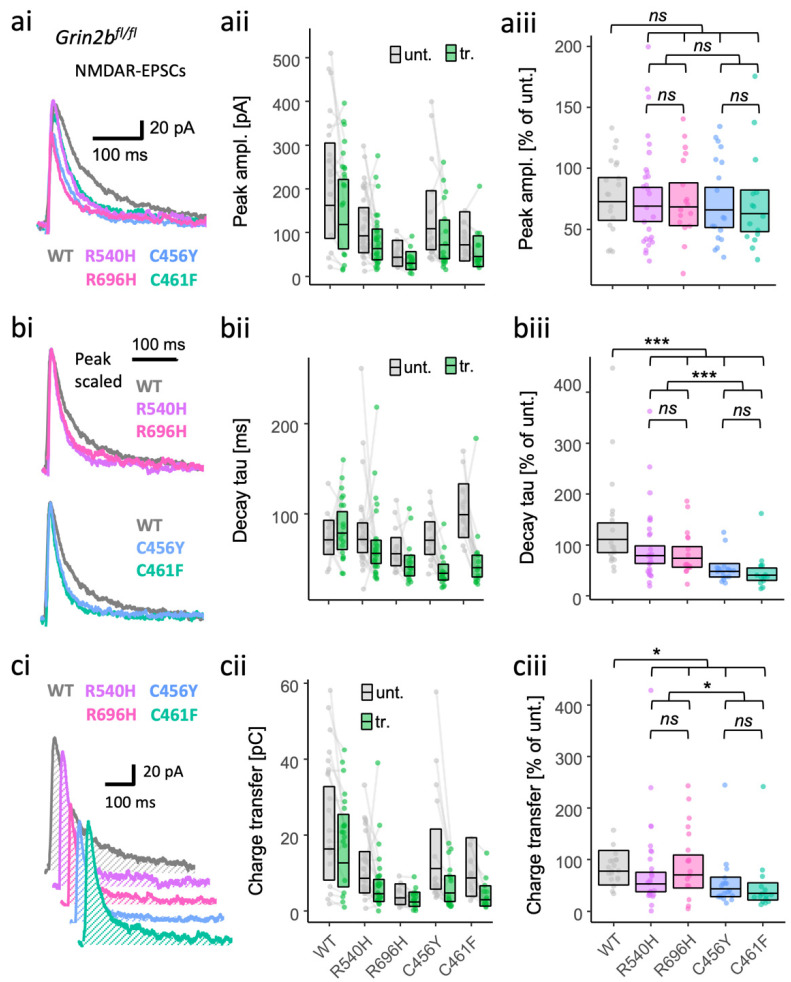
Figure 2.
Gain- and loss-of-function GluN2B mutants are both associated with more rapidly decaying NMDA-EPSCs in GluN2B knockout neurons. NMDA-EPSC+20 mV peak amplitudes (a), decay time constants (b) or charge transfer (c) in Grin2bfl/fl (untransfected) neurons and Grin2b−/− neurons rescued with human GluN2B WT, GoF (R540H and R696H) or LoF (C456Y or C461F) mutants (transfected). (a–c) (i) Representative NMDA-EPSCs (average of 10 sweeps) from transfected CA1 neurons; (ii) data points of measurements made in individual neurons, matched data points, for simultaneously recorded untransfected and transfected neurons, are connected by a line; (iii) response ratios (transfected/untransfected) are expressed as a percentage and plotted for each pair of transfected–untransfected neurons. Crossbars in (ii) and (iii) show the estimated marginal means with 95% confidence intervals backtransformed from the linear mixed models (Figure S1.4 and S1.6–7). Hypothesis tests are orthogonal contrasts based on a priori classification of the mutations (see Table S1). Standardised effect sizes (r) for comparisons of each mutant with WT for response ratios of: (aiii) peak amplitudes were −0.02, −0.04, −0.06, and −0.08; (biii) decay taus were −0.19, −0.22, −0.42, and −0.47; and (ciii) charge transfer was −0.09, −0.04, −0.24, and −0.31, for mutants R540H, R696H, C456Y, and C461F, respectively (N = 95). ns = not significant (at α = 0.05), * = p < 0.05, *** = p < 0.001.