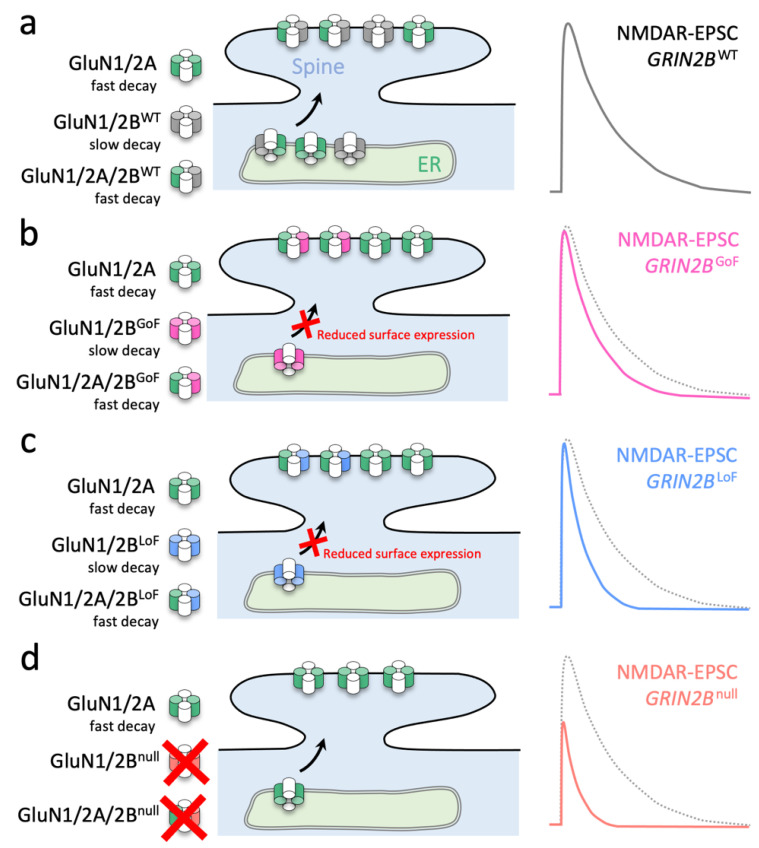
Figure 7.
Scheme summarizing how different molecular defects in the agonist binding domain of GluN2B converge to accelerate NMDA-EPSCs in CA1 neurons. (a) Schaffer collateral synapses onto CA1 neurons contain triheteromeric receptors (GluN1/2A/2B) and a small population of diheteromeric receptors (GluN1/2A and GluN1/2B). (b) GoF missense mutations in GluN2B cause GluN1/2B receptors to have more prolonged decay (Figure 1b,e), but do not traffic effectively to synapses (Figure 1b,c). Synaptic GluN1/2A/2B receptors with GoF GluN2B mutations traffic comparatively better than their GluN1/2B counterparts (Figure 2a), but the NMDA-EPSC time course is accelerated owing to the dominance of GluN2A on the deactivation of triheteromeric NMDA receptors (Figure 4b). (c) LoF missense mutations (that strongly reduce GluN2B surface expression) lead to an absence of mutant GluN1/2B receptors (Figure 1b,c), and likely also a greater representation of GluN1/2A receptors at synapses (Figure 2a). (d) Genetic deletion (i.e., the null allele) of Grin2b prevents the formation of NMDA receptors with any GluN2B subunits. GluN2A cannot fully compensate for the loss of (both) Grin2b alleles (Figure 6b). Since synapses in neurons with either GoF or LoF GluN2B missense mutations have fewer GluN1/2B diheteromers, they both exhibit more extensive inhibition of their associated NMDA-EPSCs by TCN-201 (compared to WT) (Figure 3).