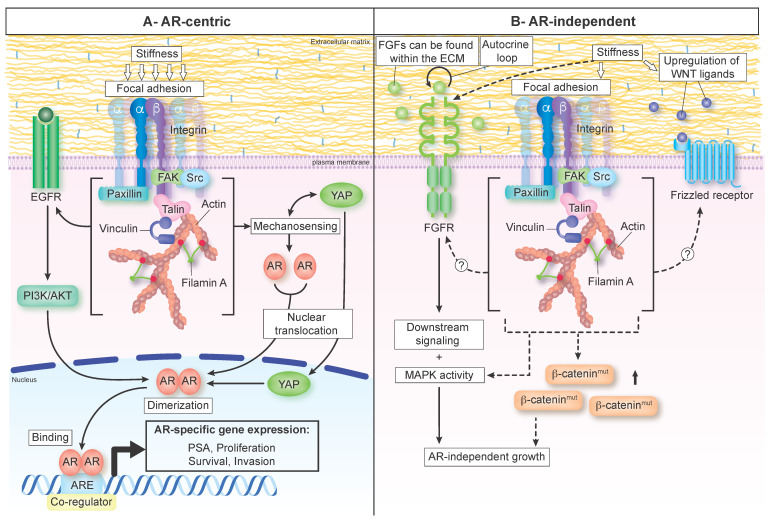
Figure 2.
Signaling pathways activated by ECM stiffness and linked to castration resistance mechanisms in prostate cancer. (A) AR-centric mechanisms. Matrix stiffness, through an excessive ECM protein deposition and their crosslink, is sensed by focal adhesions, which can in turn activate EGFR or promote YAP nuclear translocation, a mediator of the HIPPO pathway. Activated PI3K/AKT downstream of EGFR could then promote AR translocation to the nucleus, followed by its dimerization, allowing it to bind to specific regions in the DNA to trigger pro-tumorigenic cellular responses (proliferation, invasion, and survival). Nuclear YAP could also bind AR and enhance its transcriptional activity. (B) AR-independent mechanisms. Matrix stiffness sensed by focal adhesions can potentially activate FGFR or modulate the WNT pathway to drive AR-independent growth of prostate cancer. FGFs that are stored in the ECM can serve as ligands of the FGF signaling axis, and stiffness-activated FGFR will turn on downstream signaling (MAPK) to drive prostate cancer progression. Alternatively, the WNT pathway’s activation by increased stiffness can result from upregulation of the WNT ligands or increase sensitivity of the Frizzled receptor to its ligands. Increase in mutant β-catenin levels could further facilitate the crosstalk between focal adhesions and the WNT pathway. Altogether, these mechanisms could contribute to ECM stiffness-driven prostate cancer disease progression. AR: androgen receptor; ARE: androgen response element; EGFR: epidermal growth factor receptor; FAK: focal adhesion kinase; FGFR: fibroblast growth factor receptor; FGFs: fibroblast growth factors; PSA: prostate-specific antigen; YAP: Yes-associated protein.