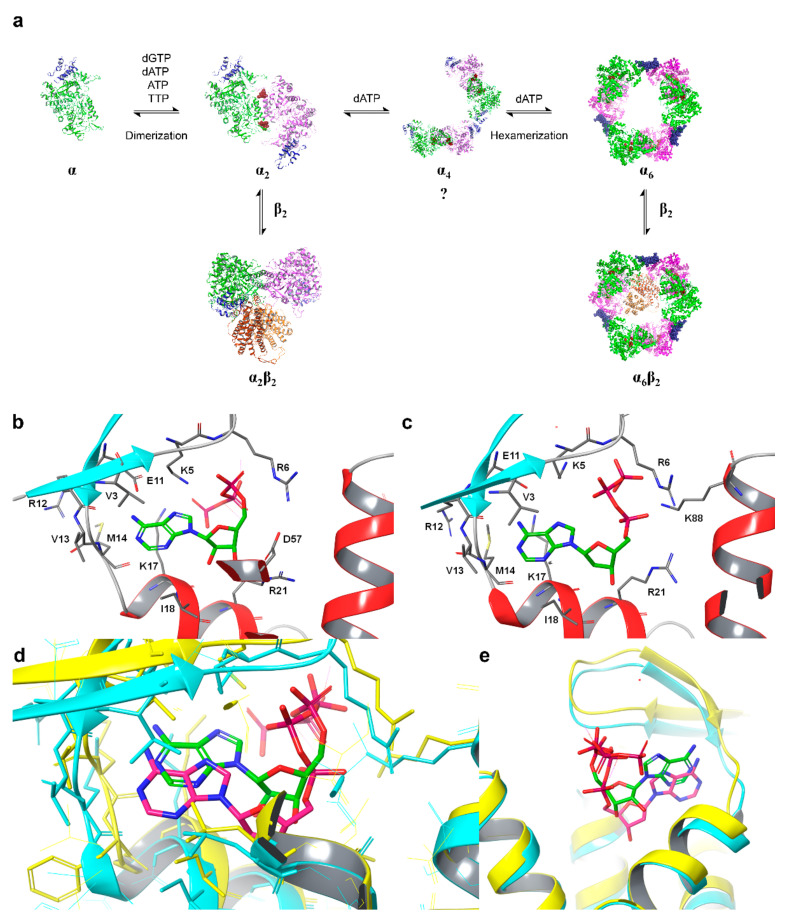
Figure 2.
(a) Model for dATP-dependent oligomerization of eukaryotic RRs. The binding of effectors to the S-site causes dimerization and the binding of dATP to the A-site causes the formation of hexamers via a hypothesized short-lived tetramer intermediate or the immediate association of three dimers to form a hexamer (question mark). Effectors bound at the S-site are maroon spheres, and the A-site is rendered as a blue ribbon. (b) ATP-hRRM1 complex. The main chain is rendered in gray (helices—red, strands—cyan), while ATP is shown in elemental green. (c) dATP-hRRM1 complex. The main chain is rendered in gray (helices—red, strands—blue), while dATP is shown in elemental green. (d) Superposition of A and B. The ATP-hRRM1 complex is rendered in yellow ribbon with ATP in elemental green. The dATP-hRRM1 complex is rendered as a cyan ribbon with dATP in elemental magenta. (e) The ATP-binding cones of hRRM1 in complex with TTP and ATP (yellow) or TTP and dATP (cyan). ATP is shown in elemental green and dATP in elemental magenta (dTTP is not shown).