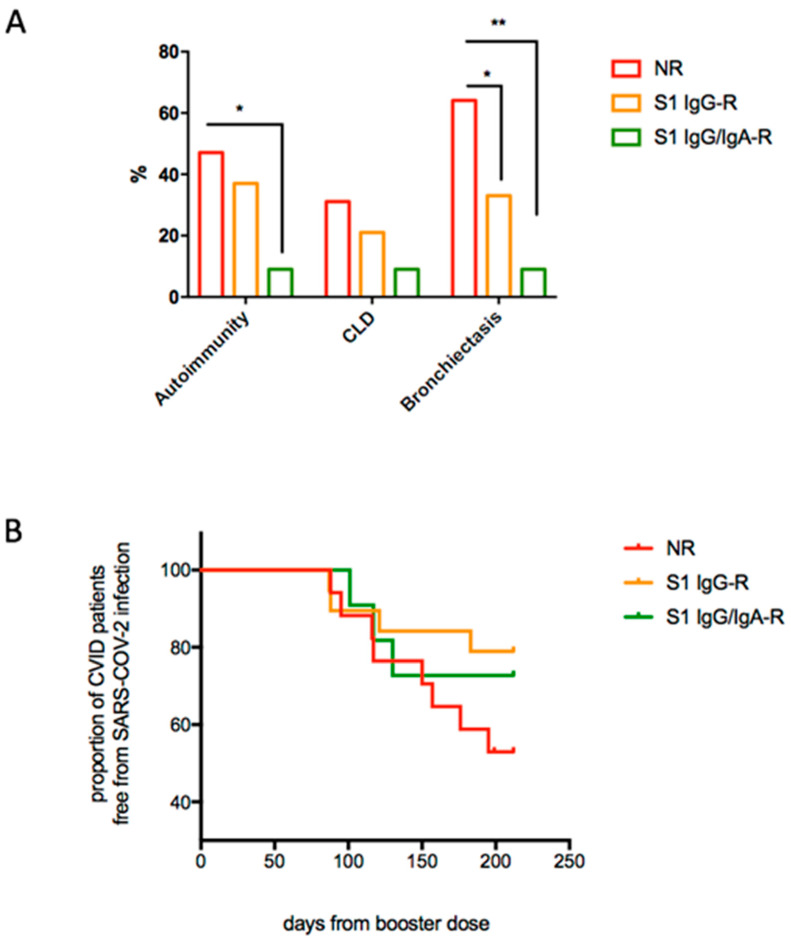
Figure 2.
CVID-associated clinical manifestations (A) and proportion of CVID patients free from SARS-CoV-2 infection after the booster dose of the mRNA BNT162b2 vaccine (B). (A): bars represented frequencies of CVID-related complications. Statistical significance was determined using a two-tailed Chi-square test. (B): Time to COVID-infection after the third dose of vaccine administration was assessed by using Kaplan–Meier product-limit estimates and based on a log-rank and Gehan–Breslow–Wilcoxon test (difference among groups not significant). * p < 0.05, ** p < 0.01 (NR n = 17; IgG-R n = 18, IgG/IgA-R n = 12).