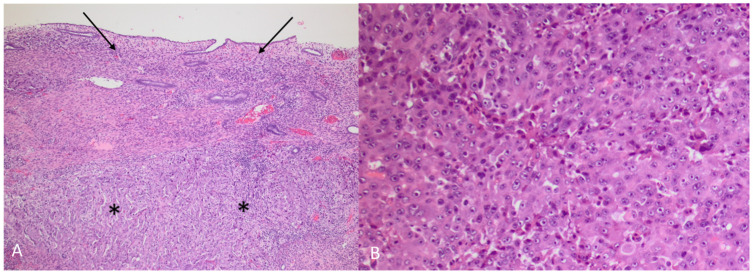
Figure 9.
(A) HAC (asterisks) growing under intact endometrium (arrows) (Hematoxylin and eosin, 5×) (B) HAC (Hematoxylin and eosin, 10×). The tumour was a poorly differentiated adenocarcinoma, primarily growing in solid sheets under a non-neoplastic endometrium. The morphology was not characteristic, and a vague trabecular pattern could only be recognized focally. The tumour cells were polygonal with enlarged, round nuclei and prominent nucleoli and with an abundant cytoplasm. There were numerous mitoses and apoptosis. Prominent blood vessel invasion was present (not shown), as previously reported by others [1,2]. Immunhistochemical staining showed an almost diffuse positive reaction in the tumour cells for AFP (α-fetoprotein) and glypican 3, with a smaller proportion of tumour cells being positive for arginase1 and HepPar1, leading to the diagnosis of HAC. The term HAC was first introduced in 1985 when gastric carcinoma with hepatic differentiation and α-fetoprotein (AFP) production was described [3]. HAC can arise from multiple other organs [4]. In a review of 261 HAC published cases, primary endometrial HAC was found to represent 4% of the documented sites that included lung (5%), gallbladder (4%), pancreas (4%), urinary bladder (3%) and ovary (10%), with stomach (63%) being the most common site [4]. The rarer sites of origin (<2%) were the retroperitoneum, oesophagus, colon, kidney, thymus, fallopian tube and adrenal glands, with the majority constituting single patient cases [4]. Most HAC tumours produce α-fetoprotein (AFP), and AFP serum levels are typically elevated at diagnosis [3,5,6,7]. The high level of AFP secretion during the foetal life, mainly by the liver and yolk sac, is dramatically reduced at birth [8]. An increase of serum AFP after birth is often associated with HCC, yolk sac tumours and benign liver diseases, such as hepatitis [4,8,9]. Although AFP secretion by HAC is a characteristic feature for this group of tumours, there are reports of HAC without AFP production [10,11]. Conversely, there are reports of AFP producing endometrial adenocarcinoma without apparent hepatoid differentiation [12,13]. Other primary uterine neoplasms such as carcinosarcoma and papillary adenocarcinoma have been shown, in rare cases, to have an AFP-secreting hepatoid component [14,15,16,17]. S-AFP has a half-life time of four to six days and constitutes a convenient tumour marker in HAC for diagnosis, evaluation during treatment and detection of recurrent disease. Other tumour markers like CEA, CA-125 and CA 19-9 may vary from normal to slightly elevated in primary uterine HAC [12,13,18,19,20]. Primary endometrial HAC is most common in elderly post-menopausal women with a median age of 64 years at the time of diagnosis [4,15]. The most common symptom is abnormal vaginal bleeding [2,7,18,19,21]. Most tumours are histologically high-grade, with an advanced clinical stage at presentation with a typically aggressive clinical course [15]. Microscopically, they are poorly differentiated adenocarcinomas proliferating in solid sheets or in a trabecular or cord-like arrangement, sometimes with microglandular or canalicular areas. Prognosis is poor, with a reported median survival of eight months [4]. Endometrial HAC metastasis has previously been reported in lymph nodes, lungs and the cervix [2,6,7,19,22].