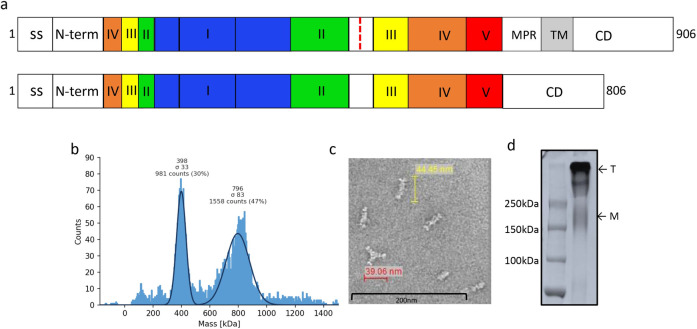
Fig 2.
CMV gB protein purification and characterization: (a) Schematic representation of full length and modified CMV gB protein. Antigenic domains (I-V), membrane proximal region (MPR), transmembrane (TM) region and furin cleavage site (as red dotted line) are indicated. The MPR and TM regions were deleted and furin cleavage site was mutated in the gB protein used for vaccine formulation. Schematic representation of gB protein was adapted from Ref. 35 and 36. (b) Mass photometry analysis of CMV gB protein showing trimer and dimer of trimer form of gB protein; molecular weight for trimeric gB was 398 kDa and 796 KDa for dimer of trimer. (c) Negative stain electron micrographs of CMV gB protein representing oligomeric forms of gB protein. Scale bar, 200 nm. (d) SDS-PAGE analysis of purified CMV gB protein under non-reducing condition. Protein migrates as a trimer or dimer of trimer (T). A small fraction of monomer (M) is also visible on SDS-PAGE.