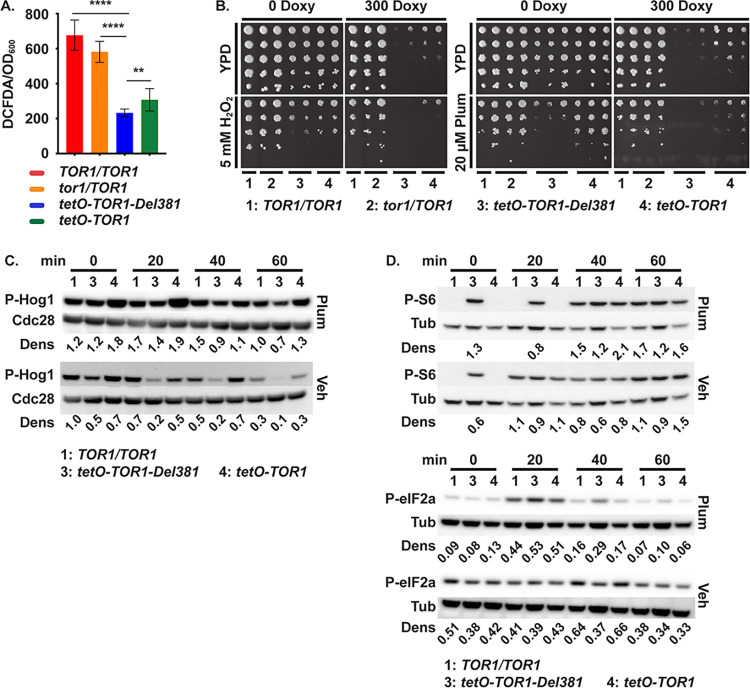
Fig 5. Tor1 N-terminal HEAT repeats were required for oxidative stress responses.
A. DCFDA-detectable ROS. Cells cultured overnight in YPD were diluted in SC medium (LoFlo) at OD600 0.5 and fluorescence intensity was determined after staining cells with 50 μM DCFDA for 90 minutes. **** is p<0.0001; ** is p = 0.0079; error bars show SD of 3 biological replicates. (TOR1/TOR1, JKC1713; tor1/TOR1, JKC1347; tetO-TOR1-Del381, JKC1441; tetO-TOR1, JKC1549). B. Dilutions of cells of indicated genotypes were spotted on YPD medium with or without 300 ng/ml doxycycline (300 Doxy), oxidative stress was induced with 5 mM H2O2 or 20 μM Plumbagin (Plum). (TOR1/TOR1, JKC1361; tor1/TOR1, JKC1345, JKC1346, JKC1347; tetO-TOR1-Del381, JKC1442, JKC1445, JKC1441; tetO-TOR1, JKC1543, JKC1546, JKC1549). C, D. Cells of indicated genotypes were pre-grown in YPD medium with 5 ng/ml doxycycline for 3.5 h (Time 0) and then diluted into fresh YPD medium with 5 ng/ml doxycycline with either 10 μM Plumbagin (Plum) or DMSO as vehicle (Veh). Total protein extract was probed with antibody to phosphorylated Hog1 (P-Hog1) and the PSTAIRE antigen of Cdc28 as loading control (C), or with antibody to phosphorylated Rps6 (P-S6) and eIF2a (P-eIF2a), and tubulin (Tub) as loading control (D). Dens: signal intensity ratio of P-Hog1 to Cdc28 (C) or P-S6 or P-eIF2a to Tub (D) (TOR1/TOR1, JKC1713 for C and JKC1361 for D; tetO-TOR1-Del381, JKC1441; tetO-TOR1, JKC1549). Same samples were run on separate gels to detect either P-S6 or P-eIF2a in panel D.