Purpose:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is regarded as a multisystemic disease. Patients with preexisting cardiovascular disease have an increased risk for a more severe disease course. This study aimed to investigate if a higher degree of coronary artery calcifications (CAC) on a standard chest computed tomography (CT) scan in mechanically ventilated patients was associated with a more severe multiorgan failure over time.
Materials and Methods:
All mechanically ventilated intensive care unit patients with SARS-CoV-2 infection who underwent a chest CT were prospectively included. CT was used to establish the extent of CAC using a semiquantitative grading system. We categorized patients into 3 sex-specific tertiles of CAC: lowest, intermediate, and highest CAC score. Daily, the Sequential Organ Failure Assessment (SOFA) scores were collected to evaluate organ failure over time. Linear mixed-effects regression was used to investigate differences in SOFA scores between tertiles. The models were adjusted for age, sex, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, cardiovascular risk factors, and chronic liver, lung, and renal disease.
Results:
In all, 71 patients were included. Patients in the highest CAC tertile had, on average, over time, 1.8 (0.5-3.1) points higher SOFA score, compared with the lowest CAC tertile (P=0.005). This association remained significant after adjustment for age, sex, and APACHE II score (1.4 [0.1-2.7], P=0.042) and clinically relevant after adjustment for cardiovascular risk factors (1.3 [0.0-2.7], P=0.06) and chronic diseases (1.3 [−0.2 to 2.7], P=0.085).
Conclusion:
A greater extent of CAC is associated with a more severe multiorgan failure in mechanically ventilated coronavirus disease 2019 patients.
Key Words: computed tomography, coronavirus disease 2019, multiorgan failure, coronary calcium, Sequential Organ Failure Assessment score, coronary artery disease
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was initially thought to mainly affect the pulmonary system.1,2 Nowadays, it is recognized as a multisystemic disease whereas patients with comorbidities are at increased risk of developing severe disease.3–6 Up to 14% of infected patients had severe disease with hypoxia requiring hospitalization, of whom 5% required admission to the intensive care unit (ICU) and mechanical ventilation.7
In the ICU, the Sequential Organ Failure Assessment (SOFA) score is widely used to evaluate a patients’ organ function during admission as SOFA is designed to capture changes in clinical status over time. The SOFA score includes components reflecting pulmonary, cardiovascular, hepatic, coagulation, renal, and neurological functions.8,9 Previous studies have shown that a decrease in score is associated with improved survival in mechanically ventilated coronavirus disease 2019 (COVID-19) patients, irrespective of existing comorbidities.5 In contrast, an increasing SOFA score indicates worsening of organ function and is associated with increased morbidity and mortality.
Patients with COVID-19 and preexisting cardiovascular disease (CVD) tend to have a more severe disease course,10 and serial SOFA scores are particularly suitable for such evaluation over time in a pandemic. Early identification of patients with a high risk of developing multiorgan failure and death is needed to aid clinical decision-making, to tailor patient management, and to recognize patient categories that might not benefit from ICU treatment at all.
In COVID-19 patients, coronary artery calcification (CAC), as detected on computed tomography (CT) is associated with a worse outcome.11–13 However, most studies have a cross-sectional design and do not include mechanically ventilated patients. Thus, whether a higher degree of CAC is associated with a worse SOFA score over time in this population, irrespective of preexisting cardiovascular risk factors, is unknown.
We hypothesize that a higher degree of CAC is associated with a worse disease course reflected by a higher SOFA score over time. In addition, this association is independent of patient characteristics, disease severity, cardiovascular risk factors, and comorbidity.
Thus, the aim of the present study was to investigate whether a higher degree of CAC, as an integrated quantification tool of cardiovascular risk, is associated with more severe multiorgan failure over time in mechanically ventilated patients with COVID-19. Quantifying the extent of CAC could identify patients at risk for multiorgan failure, which is associated with a worse outcome.
MATERIALS AND METHODS
Patient Population
The Maastricht Intensive Care COVID (MaastrICCht) cohort study design has been described more extensively elsewhere.14,15 Briefly, this prospective cohort study was executed in a patient population admitted to the ICU of the Maastricht University Medical Centre+ (Maastricht UMC+). During the COVID-19 pandemic, the number of ICU beds was rapidly upgraded from 27 to 64 beds. The study was designed to foster other datasets and registries according to the FAIR data principle in collaboration.14 The local Institutional Review Board (Medisch Ethische Toetsings Commissie [METC] 2020-1565/300523) of the Maastricht UMC+ approved the study, which was performed based on the regulations of Helsinki. During the pandemic, the board of directors of Maastricht UMC+ adopted a policy to inform patients and ask for their consent to use the collected data for research purposes. The study is registered in The Netherlands Trial Register (registration number NL8613) and was written following the STrengtening and Reporting of Observational studies in Epidemiology (STROBE) guideline.16
The MaastrICCht cohort included all patients with COVID-19 infection and respiratory insufficiency requiring mechanical ventilation, who were admitted in the first wave from March 25 until the June 23, 2020. A positive COVID-19 case was defined as follows: 1 polymerase chain reaction test positive for COVID-19 and/or a chest CT strongly suggestive of COVID-19 infection, indicated by a COVID-19 Reporting and Data System (CO-RADS)-score of 4 to 5, scored by a radiologist and no alternative diagnosis17–20). Patients were followed until the primary outcome was reached (ie, either death in the ICU or discharge from the ICU).14 For the present study, only patients who underwent a chest CT scan were included.
Imaging Protocol
All eligible patients underwent a chest CT scan either at the Maastricht UMC+ or the referral center for patients transported for logistical reasons due to the pandemic. Chest CT scans from patients transferred from elsewhere were requested and reassessed. As a result, vendors as well as scan parameters and reconstruction techniques differed between patients. Scans in our center were performed on 4 different scanners. In case of a new, clinically stable triage patient, scans were performed on a mobile CT scan unit (Alliance Medical equipped with LightSpeed 16; GE Healthcare, Milwaukee, WI), which was placed temporarily outside the hospital. When the outpatient was unstable, a more advanced system at the emergency ward was chosen (SOMATOM Definition Flash; Siemens Healthineers, Forchheim, Germany). Clinical inpatients were scanned within the Department of Radiology and Nuclear Medicine following the regular clinical pathway (SOMATOM Force; SOMATOM Definition AS (Siemens Healthineers). Tube voltage on these scanners varied between 90 and 140 kV. Additional to these scans, CTs from different hospitals throughout The Netherlands were included as well. Therefore, scan and reconstruction parameters differed. CT scans were performed in the caudocranial direction with and without the use of intravenous contrast material.
Coronary Calcium Score
CAC was graded with a semiquantitative grading system and graded on the data available on the PACS workstation (IMPAX, version 6.6.1.5003; AGFA HealthCare N.V., Mortsel, Belgium). All data were rated in consensus by 2 readers (B.M. and C.M.), experienced in cardiac imaging. The readers were blinded for patient outcome and were allowed to adjust the window level. A semiquantitative grading system was used to assess the calcifications according to their location in the left main, left anterior descending, left circumflex, and right coronary artery as 0=absent, 1=mild, 2=moderate, and 3=severe.21–23 The 4 separate scores can be summed up to get an overall grade reaching from 0 to 12,24,25 where 0 is the absence of CAC and 12 is severe calcified plaques in all coronary arteries (left main, left anterior descending, left circumflex, and right coronary artery).
Serial Outcome Variable of Multiorgan Failure: The SOFA Score
In mechanically ventilated patients with a COVID-19 infection within the MaastrICCht cohort, every component of the SOFA score was collected daily as previously described in detail.5,14 The SOFA score includes components reflecting the pulmonary, cardiovascular, hepatic, coagulation, renal, and neurological status. Each organ system component is scored as 1 of 5 categories, ranging from 0 (normal organ function) to 4 (most abnormal organ function).8 The SOFA score is the sum of the 6 organ system component scores ranging from 0 to 24 (Supplemental Table 2, Supplemental Digital Content 2, http://links.lww.com/JTI/A219). A higher score indicates worse multiple organ function and is associated with a higher morbidity and mortality.
Confounders
Comorbidities were proposed as confounders as these can be associated with organ function at baseline and the course of multiorgan failure over time.26
For the present study, in addition to age, time since intubation (days, continuous) and sex (male/female), Acute Physiology and Chronic Health Evaluation II (APACHE II) score (continuous), hypertension (yes/no), dyslipidemia (yes/no), obesity (body mass index≥30 kg/m2; yes/no), current smoker (yes/no), physician-diagnosed diabetes mellitus type 2 (yes/no), chronic lung disease (yes/no), liver disease (yes/no), and renal disease (yes/no) were considered as potential confounders. The APACHE II score is a physiologically based classification system for measuring the severity of illness in groups of critically ill patients.27 APACHE II and SOFA scores differ, although both score severity of critical illness.
Statistical Analyses
The sample characteristics were described using median and interquartile range, mean and SD, median and interquartile range, or percentages, as appropriate.
First, the cohort was categorized into sex-specific tertiles of CAC. The first tertile was the patient group with the lowest CAC score, the middle with intermediate and the third tertile with the highest CAC score. Then, baseline characteristics were compared across tertiles using the Kruskal-Wallis, 1-way analysis of variance, χ2, or Fisher exact test as appropriate.
Linear mixed-effects regression was used with a random intercept for participant and time since intubation to investigate the association between CAC and SOFA scores by computing differences in average SOFA scores between tertiles (with the lowest tertile as reference). In addition to estimating longitudinal SOFA score differences between CAC tertiles, a full longitudinal assessment requires addressing an increase/decrease in SOFA scores over time. Therefore, linear mixed-effects regression was used with a random intercept and random slope to compute average differences in the slope over time (ie, increased/decreased) between groups. When the difference in the slope over time was not statistically significant, models for average differences were presented. Specifically, we used an unstructured variance-covariance matrix and an autoregressive correlation structure of the first order for longitudinal measures.
SOFA score differences were assessed using crude sex-specific CAC tertiles (Model 1). Next, the hypothesis was subsequently challenged that a higher degree of CAC is associated with a higher SOFA score over time by adjusting for patient characteristics, admission disease severity, other cardiovascular risk factors, and comorbidity. Hence, the model was adjusted for age, sex, and APACHE II score (Model 2). In addition, the latter was adjusted for cardiovascular risk (hypertension, dyslipidemia, obesity, smoking, and diabetes mellitus type 2), as these are associated with CAC28–30 (Model 3), and finally, adjustments for chronic liver, lung, and renal disease were made (Model 4). Potential interaction of the association between tertiles of CAC and SOFA scores by time and sex was also tested, by adding an interaction term to Model 2. A 2-side P-value <0.05 and P interaction<0.05 were considered statistically significant. All the analyses were conducted using R, version 3.6.1 (R studio, Boston, MA).
RESULTS
Patient Population
In total, 94 COVID-19 patients were admitted to the ICU of our hospital during the study period. A standard chest CT was available in 71 patients (Fig. 1). Characteristics of the 71 patients who underwent a chest CT were compared with the 23 patients without chest CT (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/JTI/A218). Patients with an available chest CT scan had a longer ICU stay (18 vs. 11 d, P=0.005) and a lower arterial blood gas partial pressure of carbon dioxide of 5.3 versus 6.7 kPa (P=0.030).
FIGURE 1.
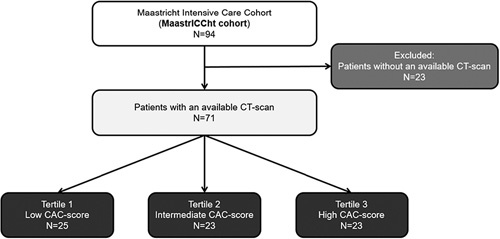
Flow diagram.
Patients were divided into 3 tertiles based on the sum of the CAC: tertile 1, score 0 to 1; tertile 2: score 2 to 6, and tertile 3: score 7 to 12. Patients within the highest CAC tertile were older (P<0.001), had more presence of diabetes mellitus type 2 (P=0.015), dyslipidemia (P=0.010), more vasopressor use (P=0.022), lower urine production (P=0.017), and lower thrombocytes (P=0.031) as compared with low and intermediate CAC tertiles (Table 1).
TABLE 1.
Demographic Characteristics, Medical History, Cardiorespiratory Indices, and Risk Indicators of Study Patients Across Tertiles of CAC
| Degree of CAC in Study Population | ||||
|---|---|---|---|---|
| Tertile 1 (n=25) | Tertile 2 (n=23) | Tertile 3 (n=23) | P for Difference | |
| Age, median (IQR) (y) | 57 (14) | 67 (11) | 73 (5) | <0.001 |
| Sex, men | 72.0 | 78.2 | 78.2 | 0.840 |
| Length of ICU stay, median (IQR) (d) | 18.0 (17.0) | 22.0 (25.0) | 15.0 (15.5) | 0.177 |
| ICU mortality | 24.0 | 30.4 | 52.2 | 0.481 |
| Height, median (IQR) (cm) | 180 (15.0) | 175 (10.0) | 173 (8.5) | 0.082 |
| Weight, median (IQR) (kg) | 90 (20.0) | 83 (12.5) | 80 (13.3) | 0.330 |
| Body mass index (kg/m2), median (IQR) | 27.8 (4.0) | 27.1 (3.5) | 27.1 (5.4) | 0.852 |
| Chronic cardiac disease | 0.0 | 0.0 | 4.3 | 0.648† |
| Chronic pulmonary disease | 4.0 | 8.7 | 13.0 | 0.508† |
| Chronic kidney disease | 4.0 | 0.00 | 0.00 | 1.000† |
| Liver disease | 0.0 | 0.0 | 4.3 | 0.648† |
| Diabetes mellitus type 2 | 8.0 | 0.0 | 26.1 | 0.015† |
| Presence of cardiovascular risk factors | ||||
| Hypertension | 20.0 | 31.8 | 47.8 | 0.121 |
| Dyslipidemia | 4.0 | 18.2 | 39.1 | 0.010† |
| Smoking | 8.0 | 9.1 | 13.0 | 0.888† |
| Obesity | 8.0 | 4.5 | 21.7 | 0.197 |
| APACHE II score, median (IQR) (points) | 15.0 (4.0) | 15.0 (4.5) | 18.0 (6.5) | 0.052 |
| SOFA score on admission, median (IQR) | 5.0 (4.0) | 6.0 (3.0) | 7.0 (4.0) | 0.211 |
| Mechanical ventilation, yes | 88.0 | 95.5 | 95.7 | 0.610† |
| Pressure control | 44.0 | 56.5 | 60.7 | |
| Pressure support | 8.0 | 4.3 | 4.3 | |
| CPAP | 36.0 | 30.4 | 30.4 | |
| FiO2 high admission, median (IQR) | 80.0 (35.0) | 70.0 (23.8) | 70.0 (25.0) | 0.163 |
| Respiration rate high on admission, median (IQR) (per min) | 30.0 (12.0) | 26.0 (3.5) | 25.0 (4.5) | 0.149 |
| Inspiratory pressure, median (IQR) (cm H2O) | 20.0 (8.0) | 24.0 (10.0) | 24.0 (9.3) | 0.258 |
| Positive end-expiratory pressure (cm H2O) | 29.3 (±5.0) | 29.2 (±1.8) | 27.0 (±4.5) | 0.513* |
| Tidal volume, median (IQR) (mL) | 497.0 (155.0) | 464.0 (114.0) | 437.0 (93.0) | 0.609 |
| Arterial blood gas PO2, median (IQR) (kPa) | 10.5 (4.5) | 9.2 (3.2) | 9.6 (2.0) | 0.440 |
| Arterial blood gas PCO2, median (IQR) (kPa) | 5.0 (2.3) | 6.2 (1.6) | 5.2 (1.5) | 0.295 |
| Arterial blood gas, median (IQR) (pH) | 7.34 (0.15) | 7.29 (0.22) | 7.29 (0.14) | 0.487 |
| Mean arterial pressure (mm Hg) | 100.4 (±14.0) | 99.7 (±13.3) | 96.8 (±13.5) | 0.629* |
| Vasopressor use, yes | 40.0 | 65.2 | 78.3 | 0.022 |
| Bilirubin, median (IQR) (µg/L) | 9.4 (5.8) | 10.5 (5.5) | 9.6 (10.8) | 0.795 |
| Dialysis, yes | 12.0 | 8.7 | 21.7 | 0.481† |
| Creatinine, median (IQR) (µmol/L) | 77.0 (30.0) | 72.0 (37.0) | 80.0 (95.3) | 0.758 |
| Urine production, median (IQR) (mL/24 h) | 1350 (1230) | 1590 (1615) | 630 (1118) | 0.017 |
| Glasgow Coma Score, median (IQR) | 15 (0) | 15 (0) | 15 (0) | 0.062 |
| Thrombocytes, median (IQR) (109/L) | 330.5 (108.5) | 374.0 (140.0) | 254.0 (131.0) | 0.031 |
Data are presented as mean (±SD) or count (%), unless indicated otherwise. Differences were tested using the independent-samples t test or Pearson χ2test, unless indicated otherwise.
One-way analysis of variance instead of Kruskal-Wallis test.
Fisher exact test instead of χ2 test due to low expected values.
CPAP indicates continuous positive airway pressure; FiO2, fraction of inspired oxygen; PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen.
Longitudinal Associations Between Coronary Calcium and Multiorgan Failure
In the crude analyses, patients in the highest CAC tertile had, on average over time, 1.8 [0.6-3.1] points higher SOFA score when compared with those in the lowest CAC tertile (P=0.005) (Table 2, Model 1). This association remained statistically significant after adjustment for age, sex, and APACHE-II score (1.4 [0.1-2.7] points, P=0.042) (Table 2, Model 2). Regression coefficients showed a higher SOFA score in the highest CAC tertile, after adjustment for cardiovascular risk factors (hypertension, dyslipidemia, obesity, smoking, diabetes mellitus type 2) (1.3 [0.0-2.7], P=0.059) (Table 2, Model 3) and adjustment for chronic liver, lung, and renal disease (1.3 [−0.2 to 2.7], P=0.085), although not statistically significant (Table 2, Model 4).
TABLE 2.
Longitudinal Association Between Coronary Calcium Score and Development of SOFA Scores Over Time
| Model | Regression Coefficient (95% CI) | P |
|---|---|---|
| Model 1: Crude | ||
| Tertile 1, degree of CAC in the study population | Reference category | |
| Tertile 2, degree of CAC in the study population | 0.9 (−0.4 to 2.1) | 0.189 |
| Tertile 3, degree of CAC in the study population | 1.8 (0.6-3.1) | 0.005 |
| Model 2: Model 1 adjusted for age and sex, APACHE-II score | ||
| Tertile 1, degree of CAC in the study population | Reference category | |
| Tertile 2, degree of CAC in the study population | 0.7 (−0.5 to 1.9) | 0.241 |
| Tertile 3, degree of CAC in the study population | 1.4 (0.1-2.7) | 0.042 |
| Model 3: Model 2 adjusted for cardiovascular risk (hypertension, dyslipidemia, obesity, smoking, diabetes mellitus type 2) | ||
| Tertile 1, degree of CAC in the study population | Reference category | |
| Tertile 2, degree of CAC in the study population | 0.5 (−0.8 to 1.7) | 0.463 |
| Tertile 3, degree of CAC in the study population | 1.3 (−0.0 to 2.7) | 0.059 |
| Model 4: Model 3 adjusted for liver conditions, chronic lung disease, and chronic kidney conditions | ||
| Tertile 1, degree of CAC in the study population | Reference | |
| Tertile 2, degree of CAC in the study population | 0.4 (−0.8 to 1.7) | 0.482 |
| Tertile 3, degree of CAC in study population | 1.3 (−0.2 to 2.7) | 0.085 |
CI indicates confidence interval.
Longitudinal regression coefficients in SOFA scores over time between CAC tertiles are reported for average differences only, as no changes (ie, increase/decrease) in SOFA scores between CAC tertiles were observed (ie, no statistically significant interaction in time since intubation and CAC tertiles were present) (Fig. 2).
FIGURE 2.
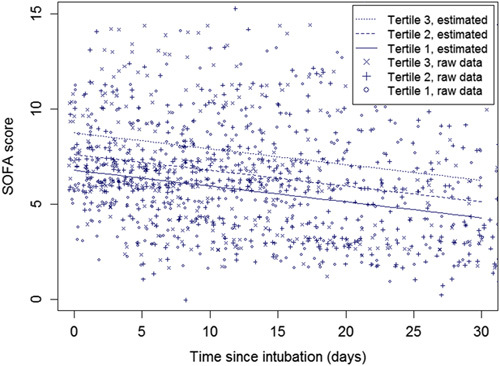
Observed and predicted SOFA scores over time for the tertiles of CAC. The figure shows that patients in the highest CAC tertile have the highest SOFA scores. In addition, SOFA scores improve gradually over time, similar for the 3 tertiles.
Furthermore, no significant interaction between sex, association of CAC tertiles, and SOFA score over time was observed (P interaction=0.712 and 0.566 for the middle and highest tertiles, respectively).
When analyzing the development of individual SOFA score components, a specific component contributed significantly more to the association between CAC and SOFA scores when compared with the other components was not identified (Table 3).
TABLE 3.
Results of Linear Mixed-effect Models: Development of Individual SOFA Component Scores
| Model 2 | Model 4 | |||
|---|---|---|---|---|
| Adjusted Regression Coefficient (95% CI) | P | Adjusted Regression Coefficient (95% CI) | P | |
| Tertile 1, degree of CAC in the study population | Reference | Reference | ||
| PaO2/FiO2 ratio | ||||
| Tertile 2 | −0.02 (−0.10 to 0.06) | 0.707 | −0.01 (−0.10 to 0.08) | 0.829 |
| Tertile 3 | −0.03 (−0.12 to 0.06) | 0.500 | −0.03 (−0.10 to 0.08) | 0.564 |
| PaO2 (kPa) | ||||
| Tertile 2 | −0.41 (−0.97 to 0.16) | 0.154 | −0.40 (−0.95 to 0.14) | 0.145 |
| Tertile 3 | −0.28 (−0.92 to 0.36) | 0.393 | −0.36 (−1.03 to 0.31) | 0.285 |
| FiO2 (%) | ||||
| Tertile 2 | 2.67 (−3.38 to 8.72) | 0.382 | 2.20 (−3.59 to 7.99) | 0.451 |
| Tertile 3 | 1.64 (−4.97 to 8.26) | 0.622 | 1.68 (−5.05 to 8.41) | 0.620 |
| SOFA cardiovascular component score | ||||
| Tertile 2 | 0.38 (−0.12 to 0.88) | 0.135 | 0.38 (−0.15 to 0.90) | 0.155 |
| Tertile 3 | 0.33 (−0.21 to 0.87) | 0.226 | 0.49 (−0.10 to 1.09) | 0.106 |
| Bilirubin (μmol/L) | ||||
| Tertile 2 | −0.75 (−5.55 to 4.05) | 0.756 | −2.17 (−6.95 to 2.61) | 0.370 |
| Tertile 3 | 2.01 (−3.25 to 7.27) | 0.449 | 1.23 (−4.31 to 6.77) | 0.660 |
| SOFA renal component score | ||||
| Tertile 2 | −0.04 (−0.73 to 0.65) | 0.913 | −0.21 (−0.89 to 0.47) | 0.537 |
| Tertile 3 | 0.62 (−0.13 to 1.38) | 0.104 | 0.40 (−0.39 to 1.19) | 0.315 |
| Glasgow Coma Score | ||||
| Tertile 2 | −0.07 (−0.20 to 0.07) | 0.343 | −0.54 (−1.60 to 0.52) | 0.317 |
| Tertile 3 | −0.03 (−0.18 to 0.12) | 0.660 | −0.54 (−1.79 to 0.71) | 0.390 |
| Thrombocytes (109/L) | ||||
| Tertile 2 | 39.5 (−28.2 to 107.2) | 0.250 | 26.6 (−42.2 to 95.4) | 0.444 |
| Tertile 3 | −53.9 (−130 to 22.2) | 0.163 | −40.1 (−121.8 to 41.7) | 0.333 |
Data are longitudinal regression coefficients that show the average difference per SOFA score component over time between CAC (CAC) tertiles, with the lowest CAC tertile as the reference category. Data are adjusted for age, sex, and APACHE II score (Model 2) and additionally for chronic liver, lung, and renal disease (Model 4).
CI indicates confidence interval; FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen.
Figure 3 shows examples of 3 patients with no, mild, and severe CAC.
FIGURE 3.
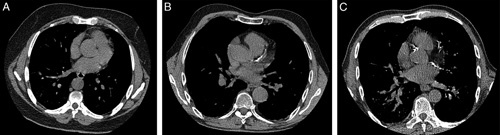
Three examples of chest CTs on CAC is of a patient with no CAC (A); a patient with CAC localized only in the left main and left anterior descending coronary artery (B); and example of a patient with extensive CAC in all the coronary arteries (C).
DISCUSSION
The present study showed that a greater extent of CAC in mechanically ventilated ICU patients with COVID-19 was associated with more severe organ failure (indicated by a higher average SOFA score), independent of age, sex, and APACHE II score. The same order of magnitude of association is observed after adjustment for cardiovascular risk factor and chronic liver, lung, and renal disease, however, not statistically significant. No changes in SOFA scores over time (ie, increase/decrease) between tertiles of CAC were observed. In addition, no significant interaction between sex and the association between SOFA score over time and the extent of CAC was found.
Previous studies have investigated the association between CAC and the outcome of COVID-19 patients. However, most studies included non-ICU patients only, used a cross-sectional design, and did not study serial data as in the present study.11,13,31 Luo and colleagues showed, in a retrospective cohort study, that a higher level of CAC was associated with more in-hospital deaths and other adverse events in COVID-19 patients. We add evidence that CAC is associated with multiorgan failure in a serial design.31 A study by Dillinger et al12 included 209 hospitalized patients with proven COVID-19, in which the presence of CAC was analyzed. CAC was associated with a worse disease course in terms of respiratory failure requiring mechanical ventilation, extracorporeal membrane oxygenation, or death. This study did not include mechanically ventilated patients and described CAC only as absent or present. In addition, Gupta et al32 have shown in a non-ICU population of 180 COVID-19 patients that CAC was associated with a higher mortality and more patients required intubation. Another single-center, retrospective, observational study applied the Agatston score to nongated chest CTs and observed more adverse events (eg, transfer to the ICU, death, or both) in hospitalized, non-ICU patients with CAC.13
The standard scoring system for CAC is the Agatston score. However, this technique is fully standardized in terms of the imaging protocol (3 mm slice thickness and a tube voltage of 120 kV) and interpretation.33 Since the chest CT scans in the present study were performed for diagnosing COVID-19 and no dedicated cardiac scans were performed at the height of the pandemic, we used a semiquantitative grading system, validated in earlier studies to be used on routine chest CTs to assess CAC.21,22,32
This study tested the hypothesis that CVD, reflected by CAC, is a potential risk factor for more severe organ failure in patients with severe COVID-19 requiring mechanical ventilation. During a pandemic, identifying patients at higher risk of a worse outcome is essential for predicting which patients will benefit from admission to the ICU. Grading CAC on a standard chest CT comes at no additional costs and may help clinicians aid decision-making. In addition, the results show that CAC may hold important prognostic information, regarding multiorgan failure as assessed by the SOFA score. Therefore, reporting CAC in all radiologic chest CT reports in COVID-19 patients might benefit clinical decision support.
This study has several strengths. First, the study is prospective by design, including many serial measurements in patients with COVID-19 infection, including a systematic data collection following a predefined protocol.14 Furthermore, CAC was assessed using a semiquantitative grading system, as validated in the literature for determining CAC in nongated chest CT scans.21,22 CAC was assessed in consensus by 2 readers experienced in cardiac imaging. In addition, we used the SOFA score in a longitudinal design. The SOFA score, in contrast to other disease severity scores such as the APACHE II score27,34 and the Simplified Acute Physiology Score (SAPS),35 was developed for serial data and thus longitudinal evaluation. Moreover, earlier research in COVID-19 patients has shown that a worse course of the SOFA score over time is associated with a worse outcome, independent of the APACHE II score.5 Last, assessing a clinical SOFA score is less complex than APACHE and SAPS scores. Therefore, it is applicable when both time and resources are scarce, such as in a pandemic situation.
Study Limitations
First, this is a single-center study with a rather small patient population, which included only mechanically ventilated ICU patients, thereby limiting the generalizability of the results to patients not in need of mechanical ventilation. Second, our study was limited to patients who had an available chest CT scan. Characteristics of these patients were similar as those without available chest CT scan, except the latter had a shorter ICU stay and a higher arterial blood gas partial pressure of carbon dioxide. Therefore, it is unlikely that selection bias had a major influence on the associations. If less severely affected patients might have had a lower degree of multiorgan failure and CVD (ie, lower CAC), the reported associations might have even been underestimated. Third, the association between CAC and SOFA score loses statistical significance after adjustment for cardiovascular risk factors and chronic liver, lung, and renal disease. However, the same order of magnitude of the clinically relevant association (1.3-point higher SOFA score in both models) suggests that these cardiovascular risk factors and chronic diseases do, at least, not fully explain the observed observations between CAC and SOFA scores.36 Furthermore, in the ICU setting, a 1.3-point difference in SOFA score is considered clinically significant, as confirmed in an earlier landmark trial.37 Fourth, the SOFA score uses a limited number of organ systems and weighting is applied to each organ score. Using a limited set of variables could lead to underestimation of the degree of organ failure in patients. However, the score is widely used, can be easily calculated at the bedside, and appears effective in detecting associations with CAC compared with its component scores. Fifth, we use a semiquantitative grading system because the CT scans performed in these critically ill patients are not dedicated cardiac CT scans with a protocol suitable for determining the Agatston score. Nevertheless, the semiquantitative grading system used in this study is validated in previous studies to be used on routine chest CTs21,22,32 and scans were evaluated by 2 readers in consensus. Finally, CO-RADS scores were not reported, as they are not related to the extent of pulmonary involvement.38 In addition, all included patients had severe respiratory failure requiring mechanical ventilation. Therefore, the severity of pulmonary findings on CTs most likely do not have a major influence on the results of the study.
In conclusion, a higher degree of CAC, scored with a semiquantitative grading system, is associated with more severe organ failure (indicated by a higher SOFA score) over time, that drives worse outcomes. This association is independent of age, sex, and APACHE II score but attenuated when correcting for cardiovascular risk factors and chronic liver, lung, and renal disease. Reporting CAC on standard chest CTs in COVID-19 patients might help guide clinical decision-making.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.thoracicimaging.com.
Footnotes
B.M. and R.G.H.D. contributed equally.
The authors declare no conflict of interest.
Contributor Information
Bibi Martens, Email: bibi.martens@mumc.nl.
Lloyd Brandts, Email: lloyd.brandts@mumc.nl.
Puck Hoitinga, Email: p.hoitinga@student.maastrichtuniversity.nl.
Fauve van Veen, Email: fauve.van.veen@mumc.nl.
Mariëlle Driessen, Email: m.driessen@mumc.nl.
Vanessa Weberndörfer, Email: vanessa.weberndorfer@mumc.nl.
Bas Kietselaer, Email: b.kietselaer@zuyderland.nl.
Chahinda Ghossein-Doha, Email: chahinda.ghossein@mumc.nl.
Hester A. Gietema, Email: hester.gietema@mumc.nl.
Kevin Vernooy, Email: kevin.vernooy@mumc.nl.
Iwan C.C. van der Horst, Email: iwan.vander.horst@mumc.nl.
Joachim E. Wildberger, Email: j.wildberger@mumc.nl.
Bas C.T. van Bussel, Email: bas.van.bussel@mumc.nl.
Casper Mihl, Email: casper.mihl@mumc.nl.
REFERENCES
- 1. Arabi YM, Murthy S, Webb S. COVID-19: a novel coronavirus and a novel challenge for critical care. Intensive Care Med. 2020;46:833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simpson S, Kay FU, Abbara S, et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA—Secondary Publication. J Thorac Imaging. 2020;35:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gavriatopoulou M, Korompoki E, Fotiou D, et al. Organ-specific manifestations of COVID-19 infection. Clin Exp Med. 2020;20:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ioannidis JPA, Axfors C, Contopoulos-Ioannidis DG. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ Res. 2020;188:109890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bels JLM, van Kuijk SMJ, Ghossein-Doha C, et al. Decreased serial scores of severe organ failure assessments are associated with survival in mechanically ventilated patients; the prospective Maastricht Intensive Care COVID cohort. J Crit Care. 2020;62:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 8. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. [DOI] [PubMed] [Google Scholar]
- 9. Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA Score, SIRS Criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317:290–300. [DOI] [PubMed] [Google Scholar]
- 10. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nai Fovino L, Cademartiri F, Tarantini G. Subclinical coronary artery disease in COVID-19 patients. Eur Heart J Cardiovasc Imaging. 2020;21:1055–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dillinger JG, Benmessaoud FA, Pezel T, et al. Coronary artery calcification and complications in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13:2468–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zimmermann GS, Fingerle AA, Muller-Leisse C, et al. Coronary calcium scoring assessed on native screening chest CT imaging as predictor for outcome in COVID-19: an analysis of a hospitalized German cohort. PLoS One. 2020;15:e0244707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tas J, van Gassel RJJ, Heines SJH, et al. Serial measurements in COVID-19-induced acute respiratory disease to unravel heterogeneity of the disease course: design of the Maastricht Intensive Care COVID cohort MaastrICCht; 2020. Available at: www.medrxivorg/content/101101/2020042720080309v1. Accessed August 19, 2020.
- 15. Tas J, van Gassel RJJ, Heines SJH, et al. Serial measurements in COVID-19-induced acute respiratory disease to unravel heterogeneity of the disease course: design of the Maastricht Intensive Care COVID cohort (MaastrICCht). BMJ Open. 2020;10:e040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 17. Prokop M, van Everdingen W, van Rees Vellinga T, et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology. 2020;296:E97–E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schalekamp S, Bleeker-Rovers CP, Beenen LFM, et al. Chest CT in the emergency department for diagnosis of COVID-19 pneumonia: dutch experience. Radiology. 2021;298:E98–E106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanfi SH, Lalani TK, Saghir A, et al. COVID-19 and its mimics: what the radiologist needs to know. J Thorac Imaging. 2021;36:W1–W10. [DOI] [PubMed] [Google Scholar]
- 20. Goyal N, Chung M, Bernheim A, et al. Computed tomography features of coronavirus disease 2019 (COVID-19): a review for radiologists. J Thorac Imaging. 2020;35:211–218. [DOI] [PubMed] [Google Scholar]
- 21. Jairam PM, Gondrie MJ, Grobbee DE, et al. Incidental imaging findings from routine chest CT used to identify subjects at high risk of future cardiovascular events. Radiology. 2014;272:700–708. [DOI] [PubMed] [Google Scholar]
- 22. Jacobs PC, Prokop M, Oen AL, et al. Semiquantitative assessment of cardiovascular disease markers in multislice computed tomography of the chest: interobserver and intraobserver agreements. J Comput Assist Tomogr. 2010;34:279–284. [DOI] [PubMed] [Google Scholar]
- 23. SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–2391. [DOI] [PubMed] [Google Scholar]
- 24. Chen L, Vavrenyuk A, Ren JH, et al. Prognostic value of coronary artery calcification identified by the semi-quantitative Weston Method in the emergency room or other hospitalized patients. Front Cardiovasc Med. 2021;8:684292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams MC, Abbas A, Tirr E, et al. Reporting incidental coronary, aortic valve and cardiac calcification on non-gated thoracic computed tomography, a consensus statement from the BSCI/BSCCT and BSTI. Br J Radiol. 2021;94:20200894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 27. Knaus WA, Zimmerman JE, Wagner DP, et al. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9:591–597. [DOI] [PubMed] [Google Scholar]
- 28. Kannel WB. Framingham study insights on diabetes and cardiovascular disease. Clin Chem. 2011;57:338–339. [DOI] [PubMed] [Google Scholar]
- 29. Kannel WB. Risk stratification of dyslipidemia: insights from the Framingham Study. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:187–193. [DOI] [PubMed] [Google Scholar]
- 30. Kannel WB, Wolf PA. Framingham Study insights on the hazards of elevated blood pressure. JAMA. 2008;300:2545–2547. [DOI] [PubMed] [Google Scholar]
- 31. Luo S, Qiu XM, Zeng XJ, et al. Coronary artery calcification and risk of mortality and adverse outcomes in patients with COVID-19: a Chinese multicenter retrospective cohort study. Chin J Acad Radiol. 2021;28:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta YS, Finkelstein M, Manna S, et al. Coronary artery calcification in COVID-19 patients: an imaging biomarker for adverse clinical outcomes. Clin Imaging. 2021;77:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hecht HS, Cronin P, Blaha MJ, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr. 2017;11:74–84. [DOI] [PubMed] [Google Scholar]
- 34. Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 35. Le Gall JR, Loirat P, Alperovitch A, et al. A simplified acute physiology score for ICU patients. Crit Care Med. 1984;12:975–977. [DOI] [PubMed] [Google Scholar]
- 36. Greenland S, Senn SJ, Rothman KJ, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31:337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hernandez G, Ospina-Tascon GA, Damiani LP, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA. 2019;321:654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abkhoo A, Shaker E, Mehrabinejad MM, et al. Factors predicting outcome in intensive care unit-admitted COVID-19 patients: using clinical, laboratory, and radiologic characteristics. Crit Care Res Pract. 2021;2021:9941570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.thoracicimaging.com.


